FDA Approves Easier-to-Use Injectable Keytruda for Cancer
TL;DR Summary
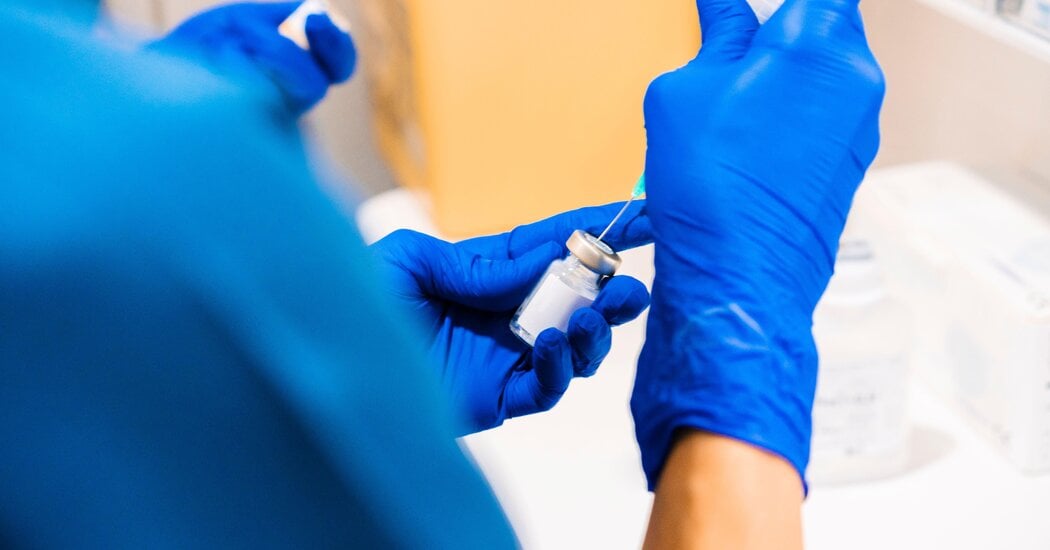
The FDA approved Merck's new subcutaneous version of Keytruda, a popular cancer drug, which offers quicker and easier administration but is likely to maintain high prices and slow the adoption of cheaper alternatives, raising concerns about increased healthcare costs.
- FDA OKs New Keytruda Shot for Cancer The New York Times
- FDA Approves Subcutaneous Pembrolizumab for Solid Tumors OncLive
- Merck wins approval of subcutaneous Keytruda, in bid to extend life of world's top-selling drug Endpoints News
- Merck Wins US Approval of Easier-to-Use Version of Cancer Drug Keytruda Bloomberg.com
- US FDA approves Merck's injectable version of blockbuster cancer therapy Keytruda Reuters
Reading Insights
Total Reads
0
Unique Readers
0
Time Saved
8 min
vs 9 min read
Condensed
98%
1,638 → 39 words
Want the full story? Read the original article
Read on The New York Times