FDA Approves Breakthrough Antibody for Infant RSV Protection
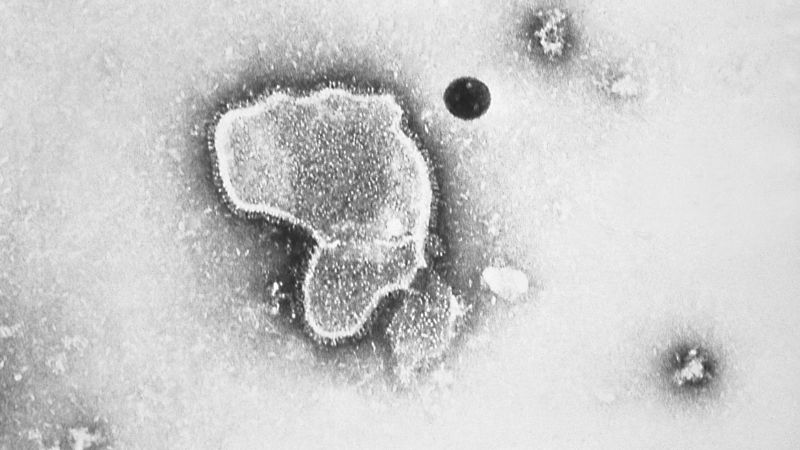
The US Food and Drug Administration (FDA) has approved nirsevimab, a new antibody called Beyfortus, to protect newborns from respiratory syncytial virus (RSV), the leading cause of hospitalization in infants under a year old in the US. Unlike vaccines, nirsevimab is a ready-made antibody that can bind to the virus and block it from infecting healthy cells. It is given as a single injection before RSV season and can also be administered to infants up to 24 months old. The FDA approval addresses the need for products to reduce the impact of RSV disease on children and the healthcare system. The Advisory Committee on Immunization Practices will next weigh in on nirsevimab's use, and the FDA is also considering approving Pfizer's vaccine for pregnant women to protect babies from RSV.
Reading Insights
0
0
6 min
vs 7 min read
90%
1,303 → 130 words
Want the full story? Read the original article
Read on CNN