"Congress and Caregivers Demand Answers: Baby Formula Recall Sparks Concerns"
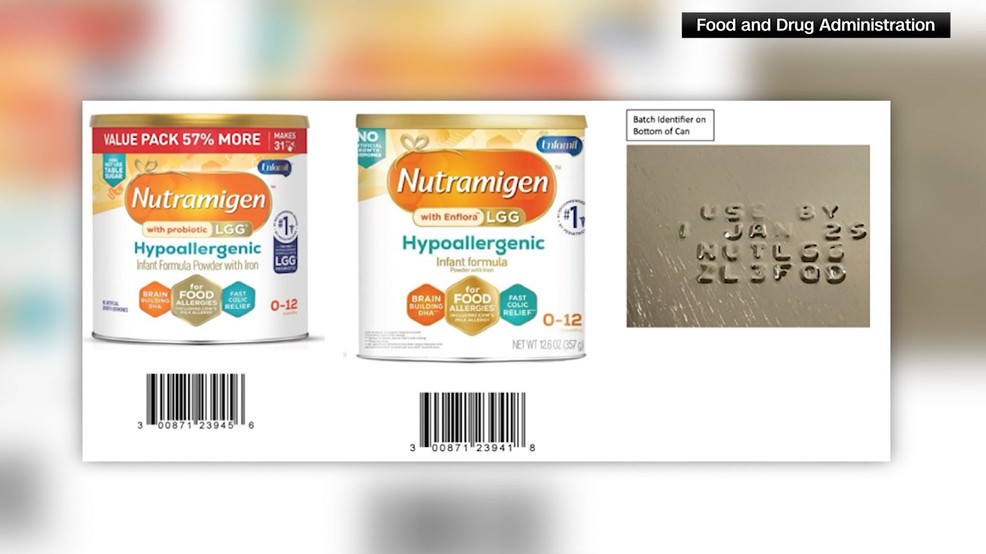
Senator Bob Casey has raised concerns and questions to Reckitt/Mead Johnson Nutrition after the recall of over 675,000 cans of Nutramigen infant powder formula due to possible contamination. This marks the second recall by Reckitt in less than a year. The recall has sparked worries about potential shortages, particularly for low-income families reliant on WIC. The FDA is currently inspecting Reckitt's Michigan plant, and the agency is in the process of overhauling its food safety division to enhance oversight and regulation of infant formula. The recall has reignited concerns about the ongoing formula shortage and its impact on caregivers and low-income families.