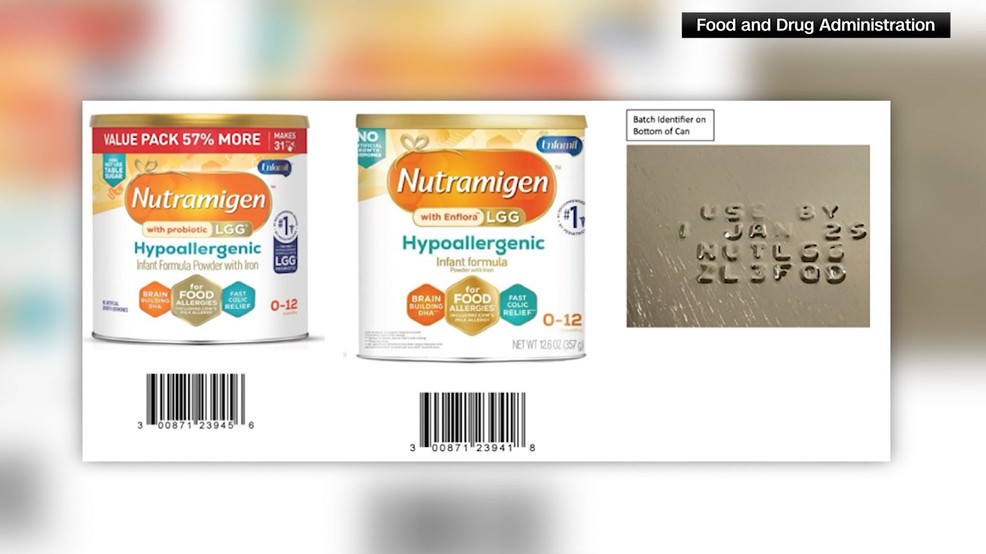
"Urgent Baby Formula Recall Issued Amid Deadly Bacteria Contamination Concerns"
The FDA has issued a warning about a recall of Reckitt/Mead Johnson Nutrition's powdered hypoallergenic baby formula due to potential contamination with Cronobacter sakazakii bacteria. Over 675,000 cans of Nutramigen Powder in specific batch codes are affected, with no reported illnesses. The formula was distributed nationwide, and while much of it is believed to have been consumed, parents are advised to check the batch codes and contact the company or their pediatrician if they have concerns.








