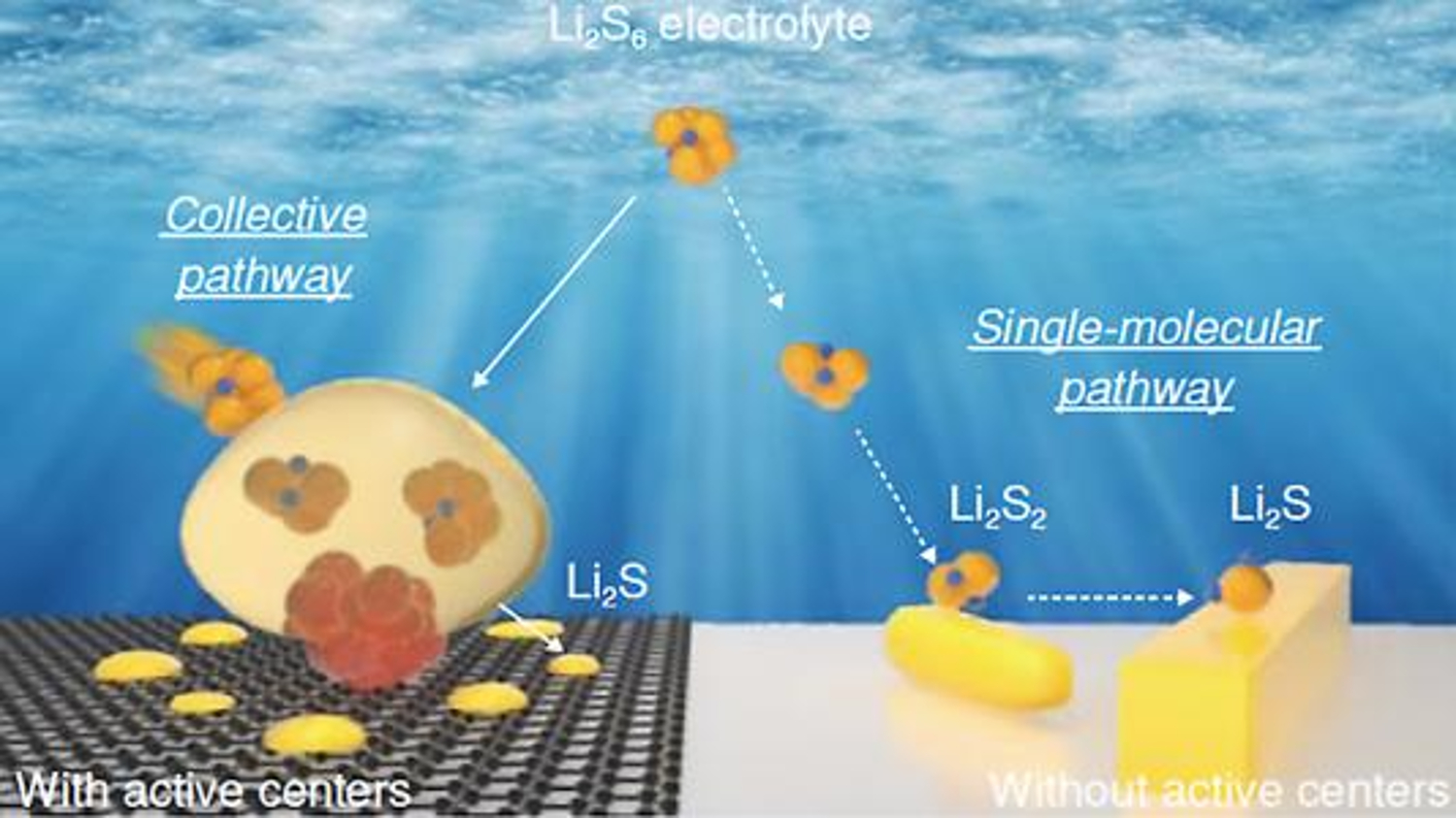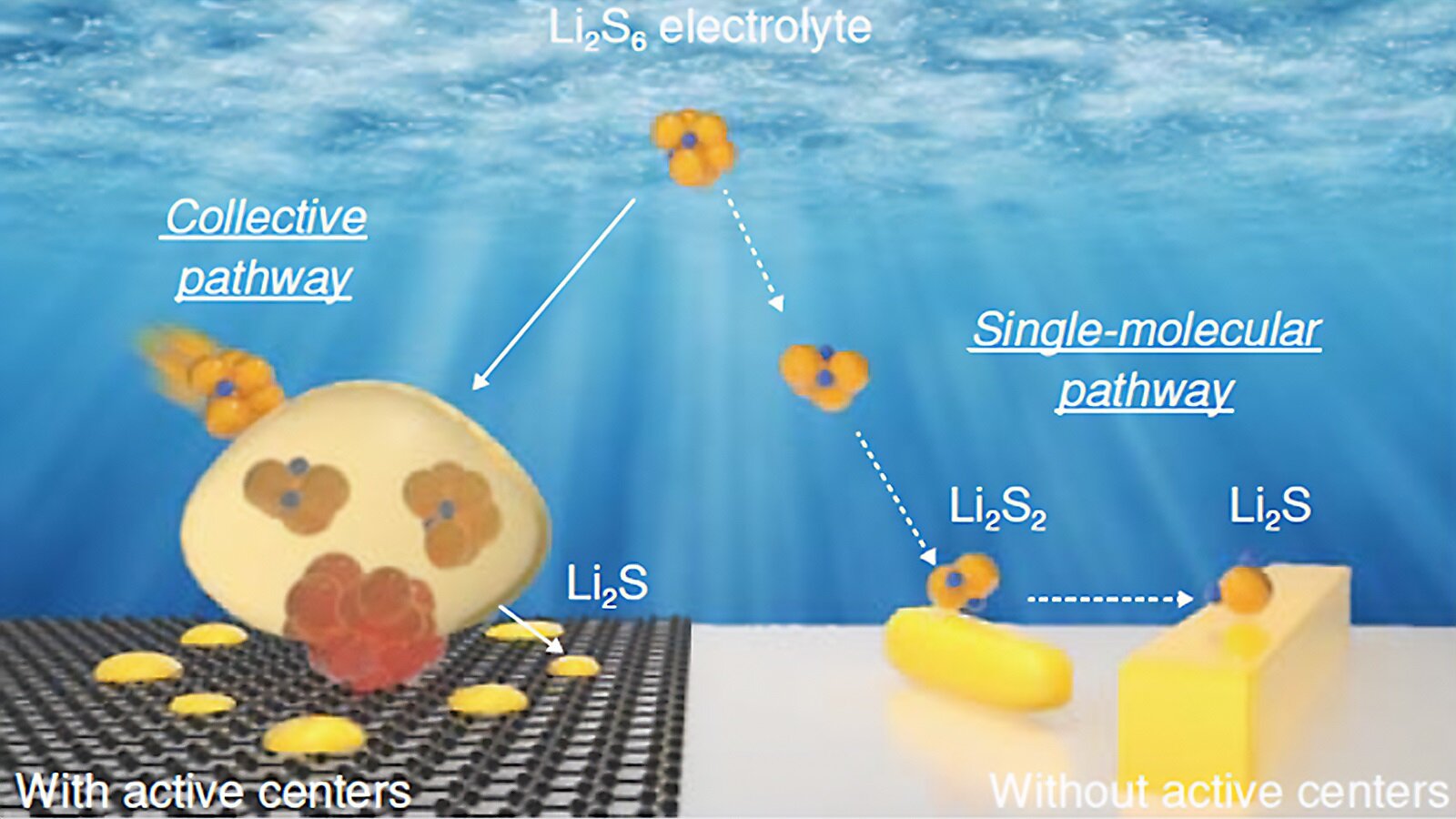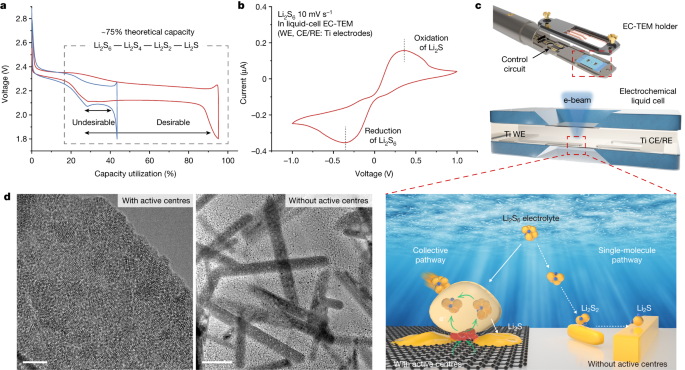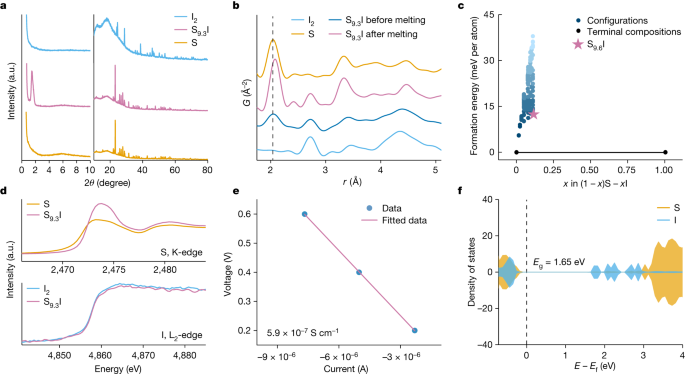
"Advancements in Healable Materials for Lithium-Sulfur Batteries"
Researchers have developed a healable and conductive sulfur iodide material for use in solid-state lithium-sulfur batteries, which are known for their high energy storage potential. The study's data is available upon request from the corresponding author. The research builds on previous work in the field of all-solid-state batteries and offers potential advancements in battery technology.