"Exploring the Dynamic Structures of the Human Intrinsically Disordered Proteome"
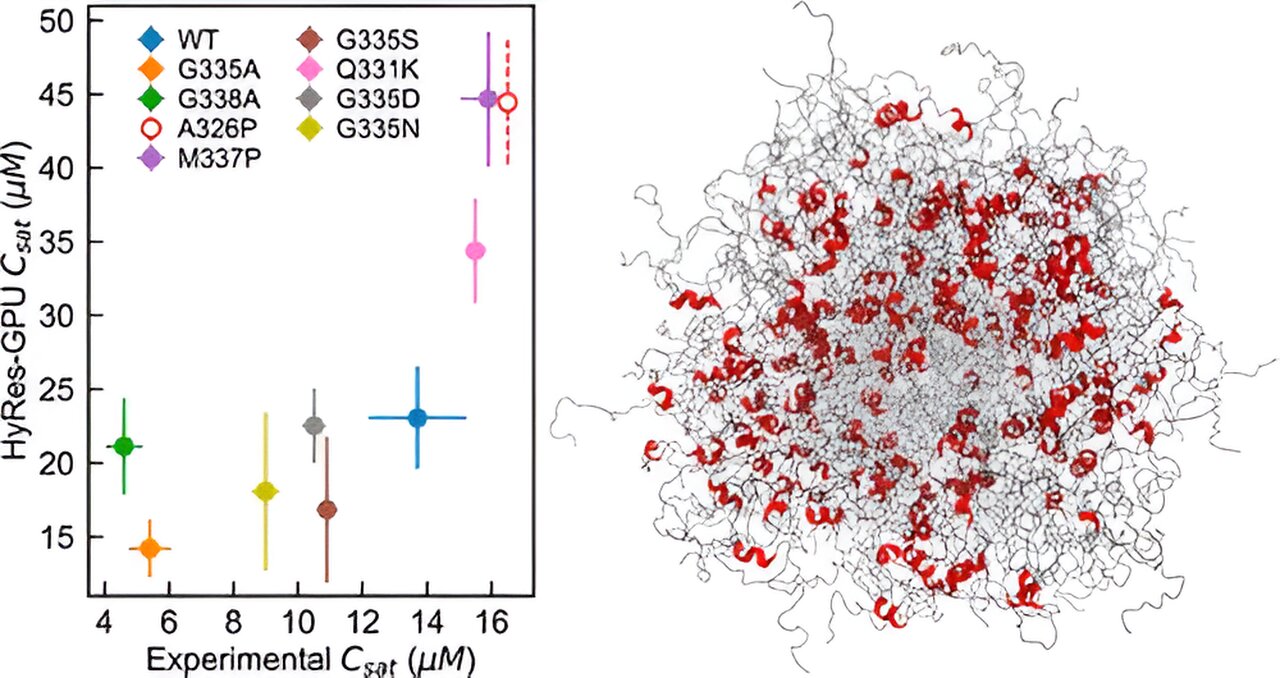
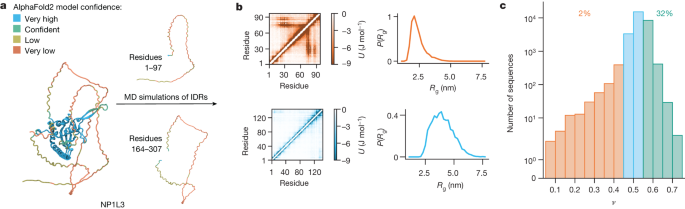
Researchers have made available simulation trajectories and conformational properties for over 28,000 intrinsically disordered regions (IDRs) of the human proteome, providing a comprehensive resource for studying these proteins. The data, which includes amino acid sequences, sequence features, and conformational properties, is accessible through an online database. Additionally, various tools and databases were utilized to gather information, and the availability of raw data to reproduce the results has been ensured. This extensive resource aims to advance the understanding of the conformational ensembles and functional implications of intrinsically disordered proteins.