Advancements in AI for Breast Cancer Screening and Diagnosis
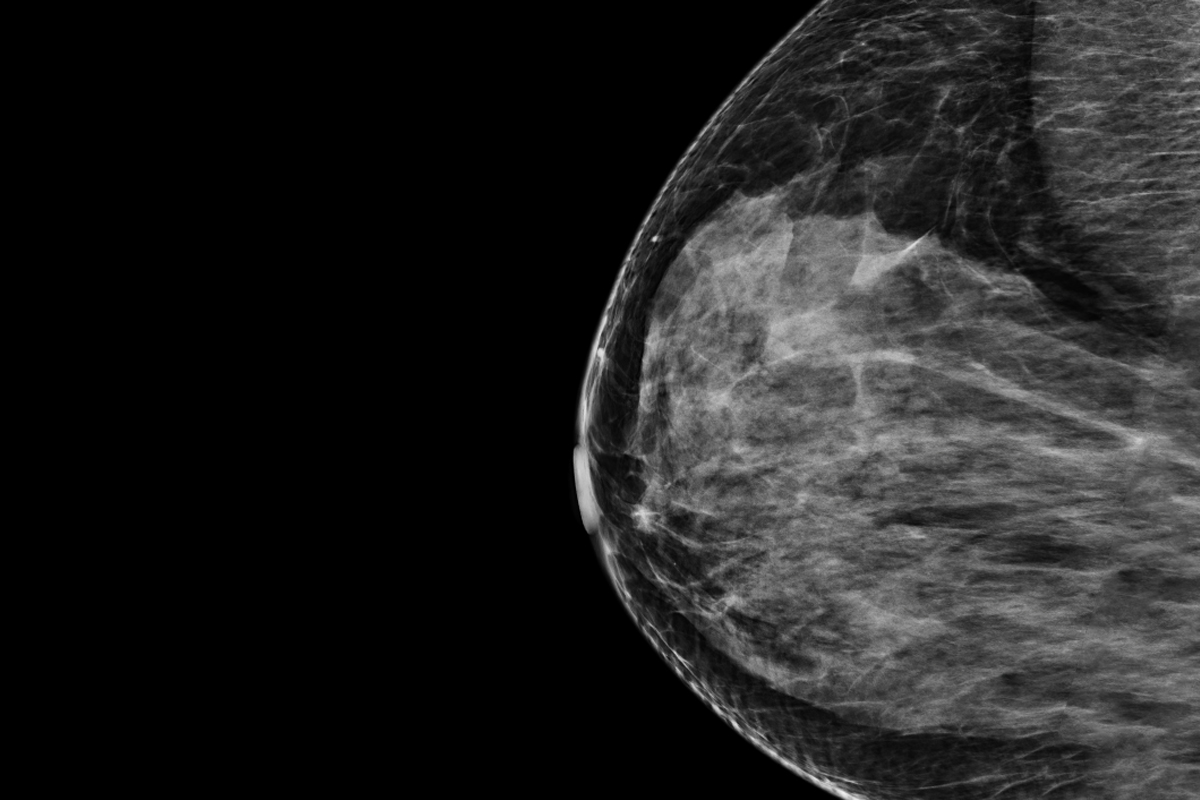
A new AI-based software developed at WashU Medicine, called Prognosia Breast, has received FDA Breakthrough Device designation for its ability to analyze mammograms and provide a more accurate five-year breast cancer risk prediction, potentially improving early detection and prevention efforts.