Stealth BioResubmits Rare Disease Drug to FDA Amid Challenges
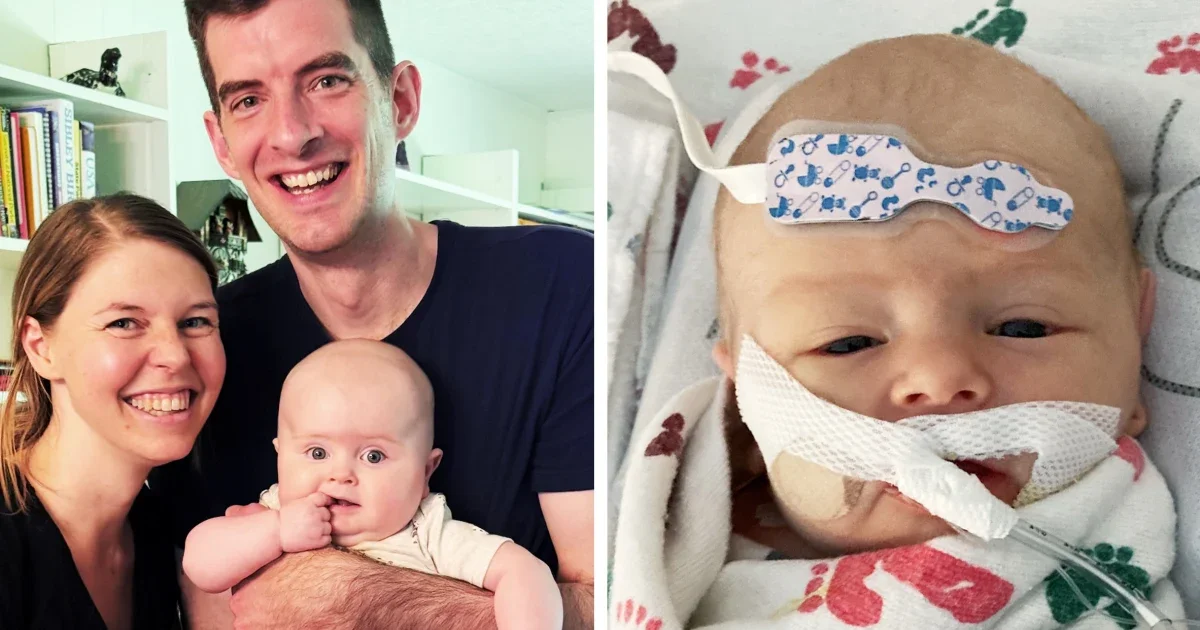
Parents of children with Barth syndrome are urgently seeking FDA approval for the experimental drug elamipretide, which has shown promise in treating the rare disease but faces delays due to manufacturing issues and regulatory hurdles, risking the lives of affected children.
