Stealth BioResubmits Rare Disease Drug to FDA Amid Challenges
TL;DR Summary
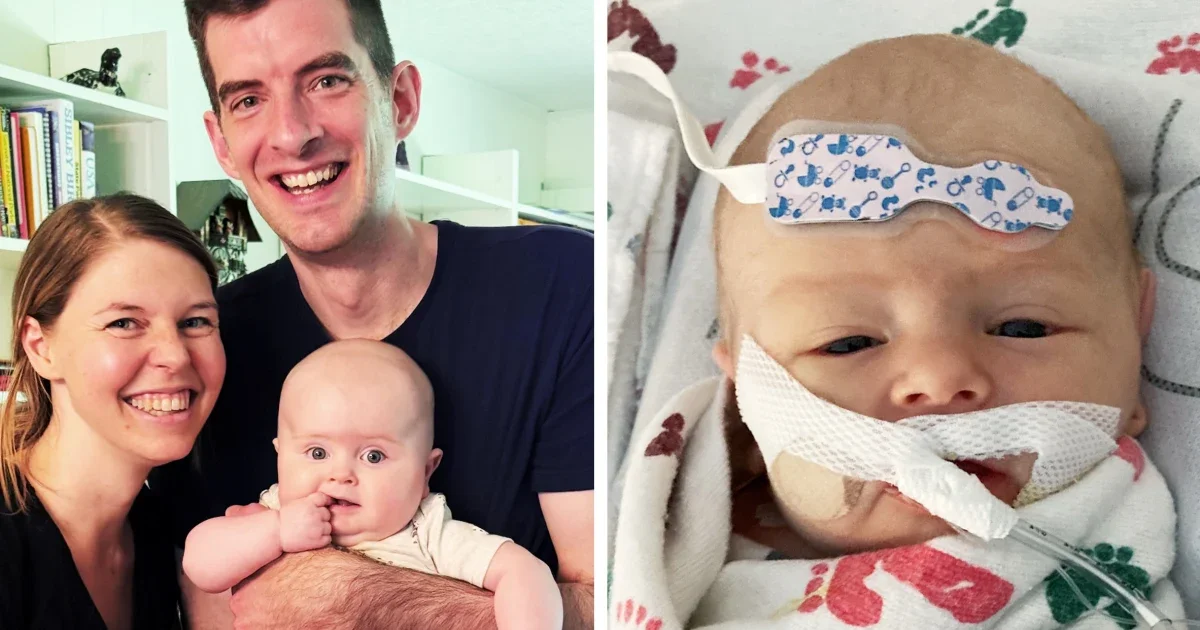
Parents of children with Barth syndrome are urgently seeking FDA approval for the experimental drug elamipretide, which has shown promise in treating the rare disease but faces delays due to manufacturing issues and regulatory hurdles, risking the lives of affected children.
- For kids with Barth syndrome, time is running out, parents say, unless the FDA acts NBC News
- STEALTH BIOTHERAPEUTICS RESUBMITS NEW DRUG APPLICATION FOR ELAMIPRETIDE FOR THE TREATMENT OF BARTH SYNDROME PR Newswire
- Stealth BioTherapeutics discloses FDA rejection letter in effort to rally support for rare disease drug statnews.com
- Stealth Bio submits third FDA application for rare disease candidate Fierce Biotech
- Stealth resubmits ultra-rare disease drug to FDA; Boehringer’s retinal deal Endpoints News
Reading Insights
Total Reads
0
Unique Readers
5
Time Saved
8 min
vs 9 min read
Condensed
98%
1,701 → 41 words
Want the full story? Read the original article
Read on NBC News