
RSV Vaccination Frequency for Seniors Over 65
For adults over 65, the RSV vaccine is recommended, but booster doses are not currently advised due to limited evidence of benefit, despite waning immunity after a year.
All articles tagged with #booster doses

For adults over 65, the RSV vaccine is recommended, but booster doses are not currently advised due to limited evidence of benefit, despite waning immunity after a year.
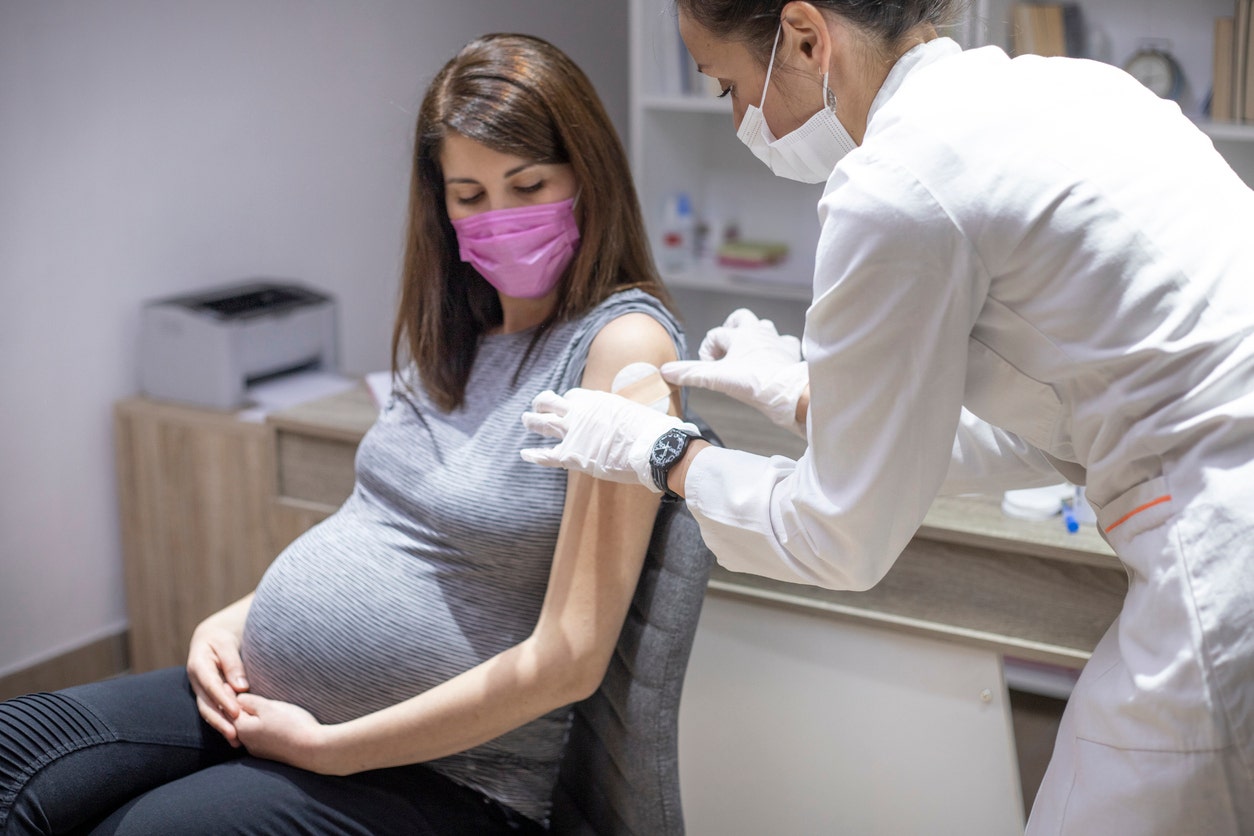
A study published in the journal Vaccine has found that getting a COVID vaccine or booster while pregnant can provide protection for both the mother and the baby. The study analyzed data from the Multisite Observational Maternal and Infant Study for COVID-19 (MOMI-VAX) and found that pregnant women who received the Pfizer or Moderna vaccines had antibodies against multiple COVID variants, including Delta and Omicron. These antibodies were also found in the cord blood of newborns, indicating transferred protection. Pregnant women face a higher risk of complications from COVID, making vaccination particularly important. The researchers recommend further studies to determine the optimal timing of vaccination during pregnancy.
A study published in JAMA Network Open analyzed 40 studies to determine how long protection from COVID-19 vaccines lasts. The researchers found that one month after receiving two doses of mRNA vaccines, AstraZeneca, or Sinovac, the vaccine effectiveness was 53% in protecting against symptoms of COVID-19. After six months, the overall effectiveness of the vaccines dropped further to 14%, and to 9% after nine months. Booster doses restored protection back to levels achieved just after the primary vaccination, but this protection waned too, at a rate similar to that after the primary series. The study suggests that COVID-19 vaccines should be given on a fixed schedule, similar to the yearly flu shot, to maintain robust protection against the virus.
A study published in JAMA Network Open analyzed 40 studies to determine how long protection from COVID-19 vaccines lasts. The researchers found that one month after receiving two doses of mRNA vaccines, AstraZeneca, or Sinovac, the vaccine effectiveness was 53% in protecting against symptoms of COVID-19. After six months, the overall effectiveness of the vaccines dropped further to 14%, and to 9% after nine months. Booster doses restored protection back to levels achieved just after the primary vaccination, but this protection waned too, at a rate similar to that after the primary series. The study suggests that COVID-19 vaccines should be given on a fixed schedule, similar to the yearly flu shot, to maintain robust protection against the virus.

The World Health Organization (WHO) has updated its guidance on COVID-19 vaccinations, stating that healthy children and teens likely do not need the vaccine. The new roadmap defines three priority groups based on the risk of severe disease and death when contracting the virus, with healthy kids between 6 months and 17 years old now deemed low priority. However, children who have compromised immune systems or existing health conditions should still get the vaccine. The guidance also calls for pregnant women to be fully vaccinated for full protection of the mother and fetus. The agency encourages countries to consider factors including disease burden, cost-effectiveness, and other health or programmatic priorities when making decisions about vaccine requirements for healthy children and teens.
The World Health Organization's vaccine experts have updated their global Covid-19 vaccination recommendations, stating that healthy children and teenagers considered low priority may not need to get a shot. The new streamlined recommendations prioritize Covid-19 vaccines for those at greatest risk of death and severe disease, with additional booster doses recommended for high-priority groups such as older people, immunocompromised people of all ages, front-line health workers, and pregnant people. The group recommends primary vaccinations and first booster doses for those at medium risk, including children and adolescents with health risks and healthy adults under the age of about 60, but does not recommend routine additional boosters. The public health impact of vaccinating healthy children and adolescents is comparatively much lower than the established benefits of traditional essential vaccines for children, such as the rotavirus, measles, and pneumococcal conjugate vaccines, according to SAGE.
WHO's Strategic Advisory Group of Experts on Immunization (SAGE) has updated its roadmap for prioritizing the use of COVID-19 vaccines, taking into account the impact of Omicron and high population-level immunity due to infection and vaccination. The roadmap continues to prioritize protecting populations at the greatest risk of death and severe disease from SARS-CoV-2 infection and maintaining resilient health systems. SAGE recommends an additional booster for high priority groups, including older adults, younger adults with significant comorbidities, people with immunocompromising conditions, pregnant persons, and frontline health workers. The medium priority group includes healthy adults and children and adolescents with comorbidities, while the low priority group includes healthy children and adolescents aged 6 months to 17 years. SAGE urges countries to base their decisions on contextual factors such as disease burden, cost-effectiveness, and other health or programmatic priorities and opportunity costs.