Insurers mandated to cover new injectable HIV prevention drugs by 2025
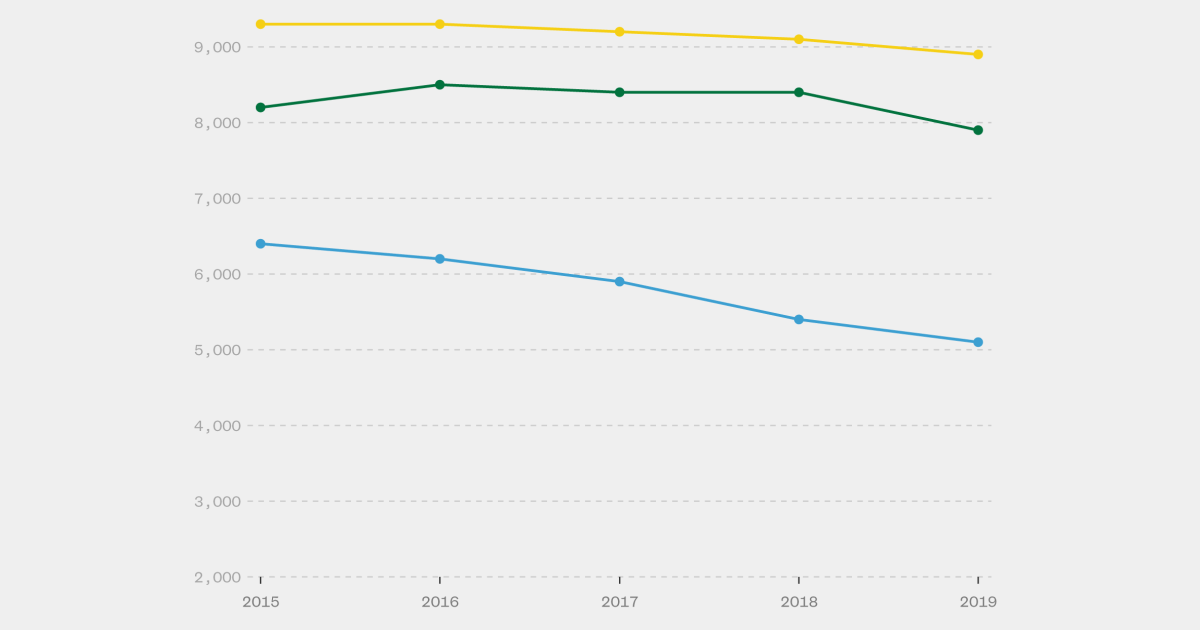
A national health task force has recommended that health insurers cover a long-acting injectable medication for HIV prevention by 2025. However, a conservative lawsuit could potentially void the coverage mandate, impacting people's ability to afford preventive interventions for various health conditions. The lawsuit, currently pending in the 5th U.S. Circuit Court of Appeals, challenges the task force's authority to dictate insurance policy. The legal battle could have broader implications beyond HIV prevention, potentially affecting coverage for other preventive interventions. The task force's endorsement of the injectable medication, Apretude, is seen as a major step in expanding access to HIV pre-exposure prophylaxis (PrEP).