Pink Viagra Returns to the Spotlight
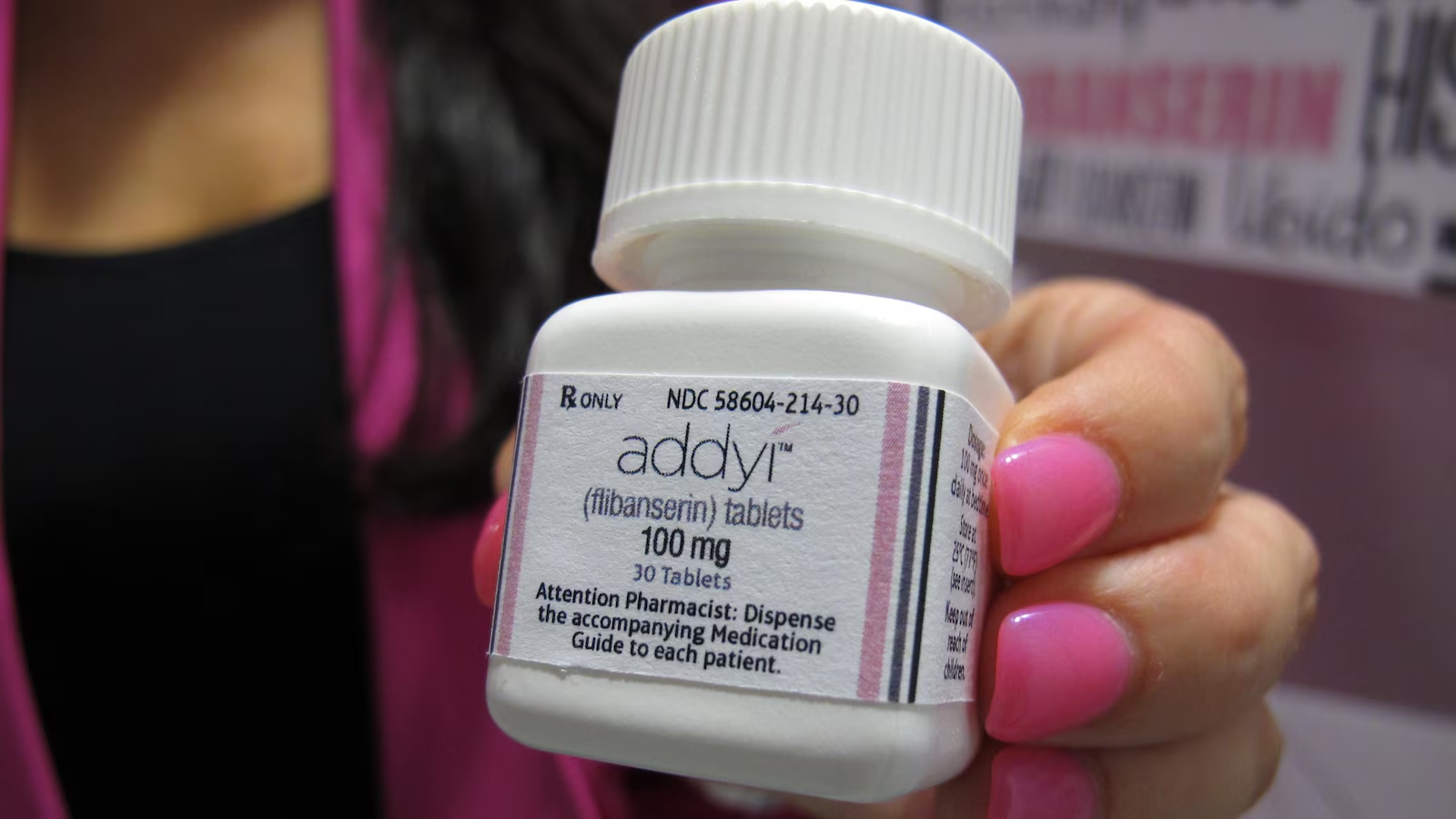
Addyi, marketed as the 'female Viagra,' was approved over a decade ago amid controversy, but it has proven to be only marginally effective with significant side effects. Despite lobbying efforts and expanded indications for older women, the drug's actual benefits are minimal, with only a slight increase in satisfying sexual events, and it remains a poor choice compared to other medications. The article criticizes the drug's approval process and marketing tactics, emphasizing that it is not a safe or effective solution for female sexual desire issues.