Ligand-tuned activation paths reveal multiple GPCR signaling states in living cells
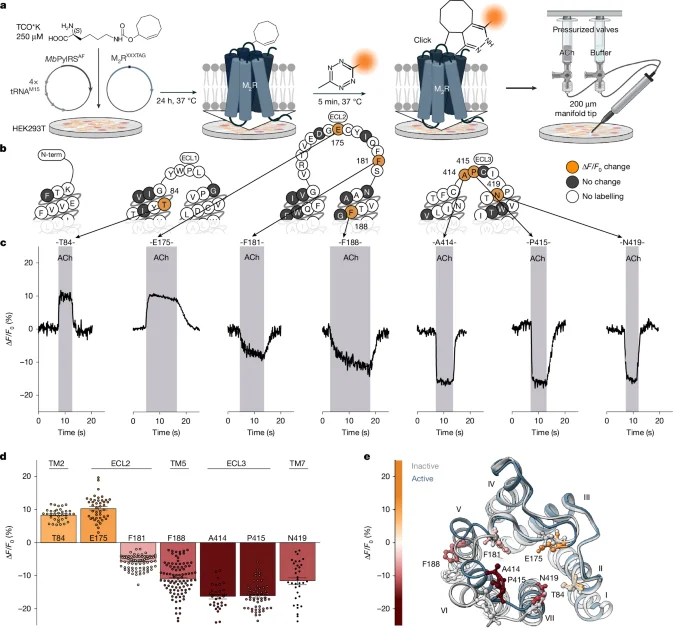
Researchers built extracellular, single-residue GPCR biosensors on the M2 muscarinic receptor using noncanonical amino acids and fast click labeling to watch real-time, ligand-induced conformational changes in live cells. They show agonists stabilize at least two distinct M2R–G-protein signaling complexes (C1 and C2) that form via ligand-specific activation trajectories, with the complex balance and trajectory dictating G-protein subtype selectivity and signaling strength. Overexpressing a nucleotide‑free Gα mutant shifts equilibria to reveal different complexes, while PTX and TRUPATH assays map GDP-bound, low-efficacy and GDP-free, high-efficacy states and their G-protein preferences. Kinetic analysis links on-rates (0.2–5 s formation) to activation trajectories, suggesting ligand-specific pathways underlie GPCR signaling diversity and offering new angles for drug discovery.
Reading Insights
0
7
73 min
vs 74 min read
99%
14,690 → 112 words
Want the full story? Read the original article
Read on Nature