"Mimicking Karyopherin Engagement: HIV Capsids and Nuclear Pore Transport"
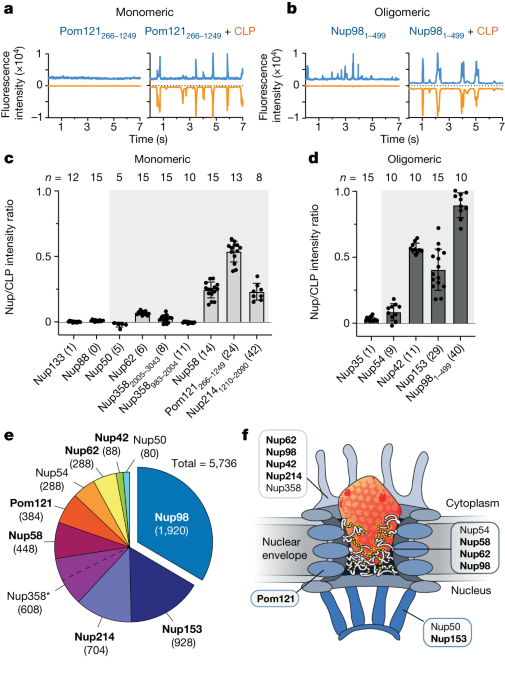
The HIV capsid, crucial for viral infection, has been found to interact with FG-nucleoporins (FG-Nups) of the nuclear pore complex (NPC) through weak but specific interactions, similar to karyopherins. The capsid's ability to bind to FG-Nups, which form the selectivity barrier of the NPC, allows it to overcome the size restriction and enter the nucleus. This interaction is mediated by an FG-binding pocket on the capsid, and mutations affecting this pocket disrupt the binding. Additionally, the capsid can enter phase-separated FG-Nup condensates, demonstrating its ability to engage with the NPC autonomously. These findings shed light on the mechanism of HIV nuclear entry and provide insights into potential targets for antiviral therapies.
Reading Insights
0
1
52 min
vs 53 min read
99%
10,438 → 111 words
Want the full story? Read the original article
Read on Nature.com