"FDA Approves Dexcom Stelo as First OTC Continuous Glucose Monitor"
TL;DR Summary
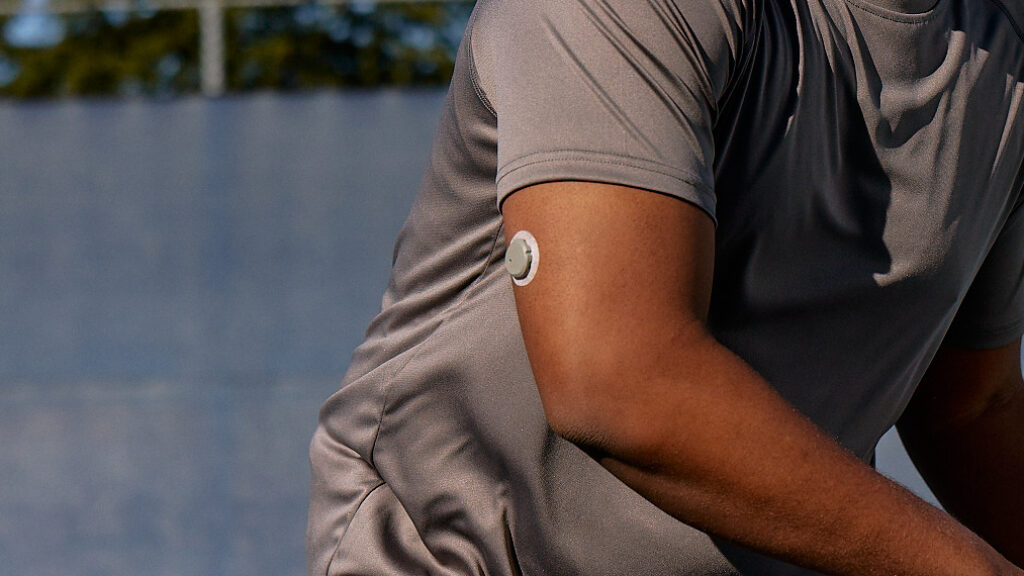
The FDA has cleared Dexcom to sell the Stelo, a continuous glucose monitor, over-the-counter to adults without insulin dependence, with the product set to launch this summer. The device is designed for people with type 2 diabetes and those without diabetes who want to monitor their blood sugar levels. Paired with a smartphone app, it provides glucose measurements every 15 minutes. This move marks a significant expansion in access to continuous glucose monitoring technology, which has become a lucrative market for companies like Dexcom and Abbott.
- First over-the-counter continuous glucose monitor OK'd by FDA STAT
- Dexcom's first-ever over-the-counter glucose monitor patch gets FDA clearance CNBC
- Dexcom Stock Pops After FDA Clears Its New Continuous Glucose Monitor Investor's Business Daily
- FDA Clears First OTC Continuous Glucose Monitoring, Dexcom Stelo Glucose Biosensor System Pharmacy Times
- FDA Clears Dexcom Stelo Sensor, First OTC Glucose Sensor in Agency History MD Magazine
Reading Insights
Total Reads
0
Unique Readers
2
Time Saved
1 min
vs 2 min read
Condensed
67%
263 → 86 words
Want the full story? Read the original article
Read on STAT