U.K. Approves Groundbreaking Gene Therapy for Sickle-Cell Disease
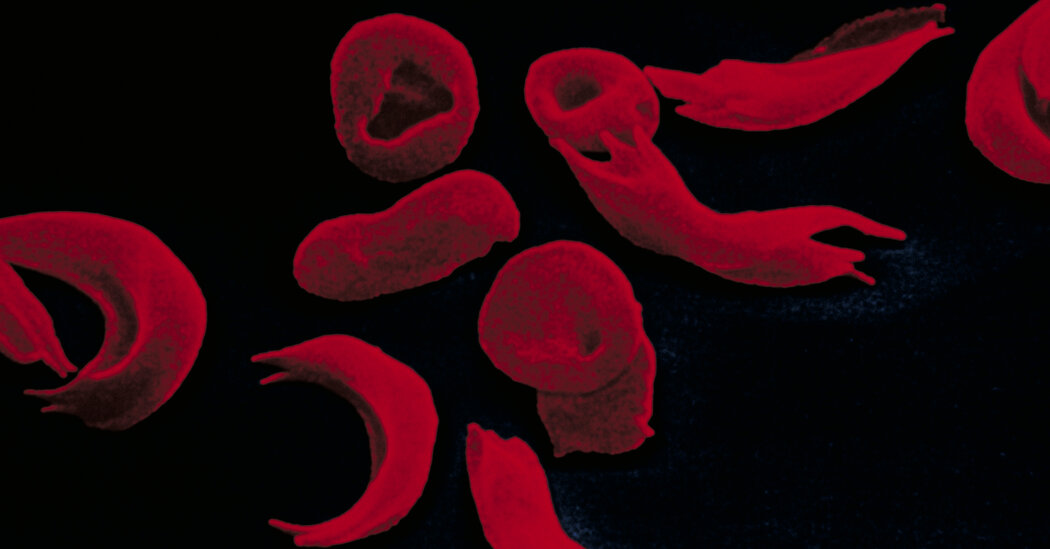
The U.K. has approved the first treatment derived from CRISPR, a gene-editing method, for sickle-cell disease and beta thalassemia. The treatment, called Casgevy, is expected to be approved by the FDA in the United States next month. The manufacturers, Vertex Pharmaceuticals and CRISPR Therapeutics, anticipate that about 2,000 patients in the U.K. will be eligible for the treatment. Sickle-cell disease affects around 100,000 Americans, mostly Black and Hispanic individuals, and causes episodes of extreme pain. The treatment relies on CRISPR to activate a gene that produces an alternative form of hemoglobin. However, the treatment is onerous and expensive, requiring intense chemotherapy and hospital stays. The price in the U.S. is expected to be millions of dollars per patient.
- Sickle-Cell Treatment Created With Gene Editing Wins U.K. Approval The New York Times
- Gravitas: Potential cure for blood disorders approved in UK WION
- World First As UK Greenlights Landmark CRISPR Gene Editing Treatment IFLScience
- U.K. approves world's first gene therapy treatment for sickle cell CBC News: The National
- View Full Coverage on Google News
Reading Insights
0
1
3 min
vs 4 min read
83%
690 → 118 words
Want the full story? Read the original article
Read on The New York Times