Gilead's New HIV Drug Gains U.S. Support and FDA Approval
TL;DR Summary
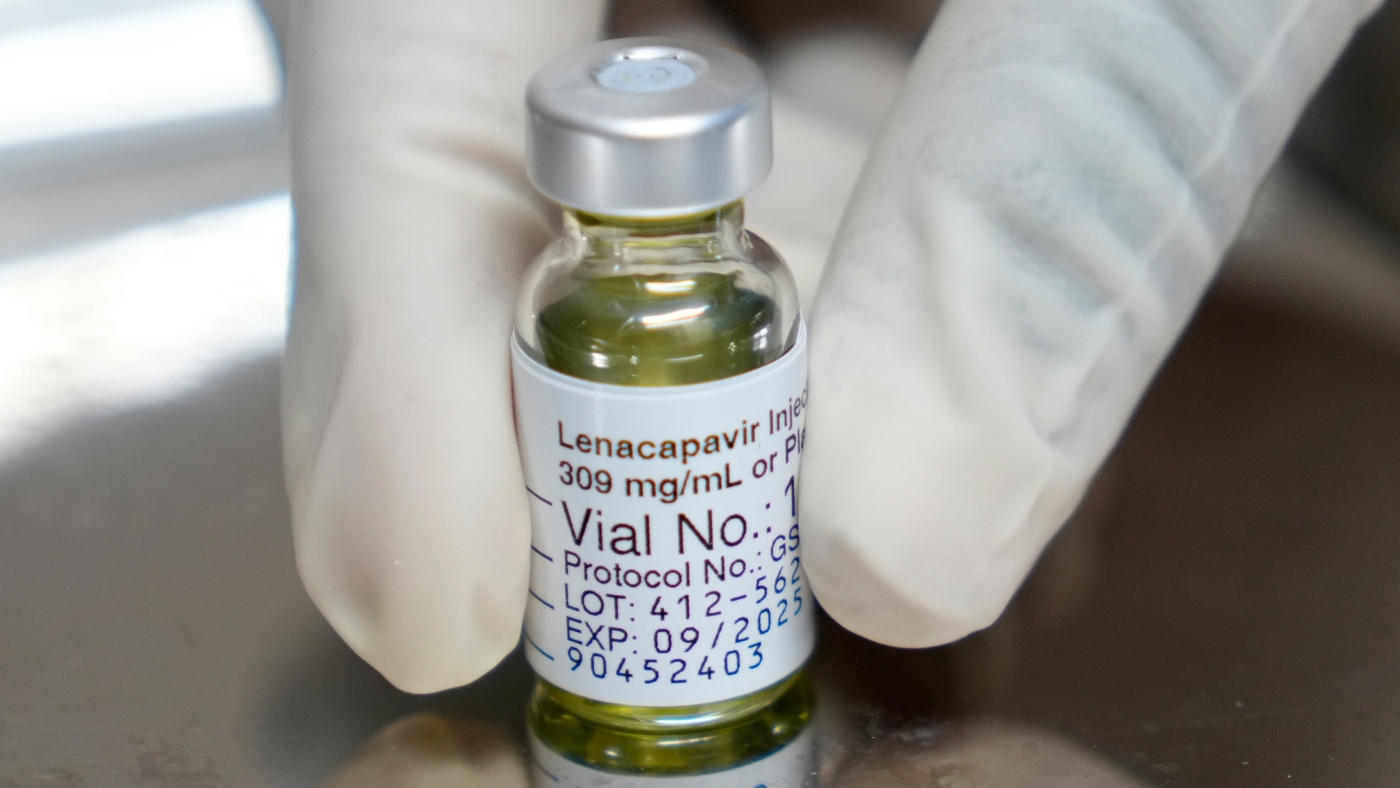
The U.S. announced support for lenacapavir, a groundbreaking HIV prevention drug requiring only biannual injections, aiming to reach 2 million people by 2028 and significantly impact the global HIV epidemic, especially in low- and middle-income countries.
- Why the medical community is thrilled by U.S. support for a 'breakthrough' HIV drug NPR
- Gilead Sciences Fierce Pharma
- Gilead joins U.S. to expand access to new HIV drug (GILD) Seeking Alpha
- Gilead Sciences, Inc. (GILD) Wins FDA Approval for World’s First Twice-Yearly HIV Preventive Therapy Yahoo Finance
- Gilead partners with PEPFAR to expand HIV prevention access Investing.com
Reading Insights
Total Reads
0
Unique Readers
9
Time Saved
7 min
vs 8 min read
Condensed
98%
1,513 → 36 words
Want the full story? Read the original article
Read on NPR