FDA Panel Recommends Approval of Needle-Free Epinephrine Nasal Spray.
TL;DR Summary
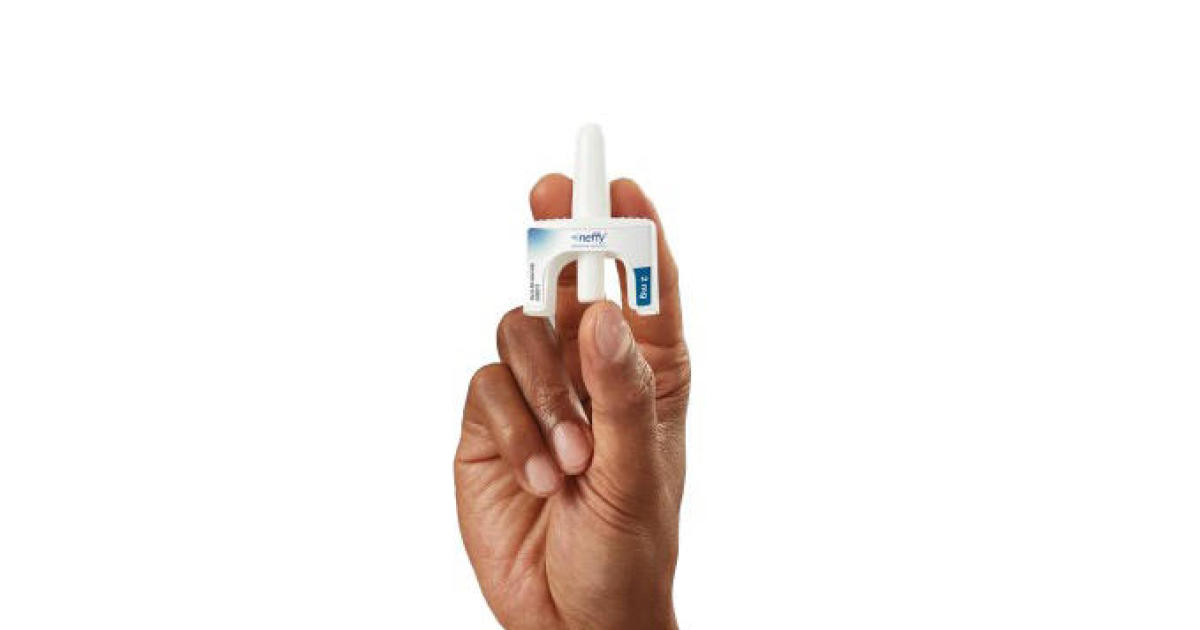
A committee of the FDA's outside advisers has voted in favor of ARS Pharmaceuticals' proposed epinephrine nasal spray, Neffy, which could soon be the first needle-free option for treating severe allergic reactions. The spray is designed to deliver a 2 milligram dose of epinephrine, which can reverse the symptoms of a life-threatening allergic reaction. The FDA is expected to issue its final verdict on whether to approve Neffy in mid-2023. If approved, the company hopes to launch the prescription spray by the end of the year.
- First epinephrine nasal spray clears key FDA hurdle, promising needle-free alternative CBS News
- FDA Panel Backs Needle-Free Epinephrine for Anaphylaxis Medpage Today
- Expert Panel Recommends FDA Approve Neffy Epinephrine Spray Allergic Living
- May 11, 2023 Meeting of the Pulmonary-Allergy Drugs Advisory Committee (PADAC) U.S. Food and Drug Administration
- FDA Staff Grapples With Absent Clinical Efficacy Data for Nasal Epinephrine Medpage Today
Reading Insights
Total Reads
0
Unique Readers
1
Time Saved
4 min
vs 5 min read
Condensed
90%
837 → 86 words
Want the full story? Read the original article
Read on CBS News