"FDA Issues Recall for CVS and Walmart Eye Ointments Over Infection Risk"
TL;DR Summary
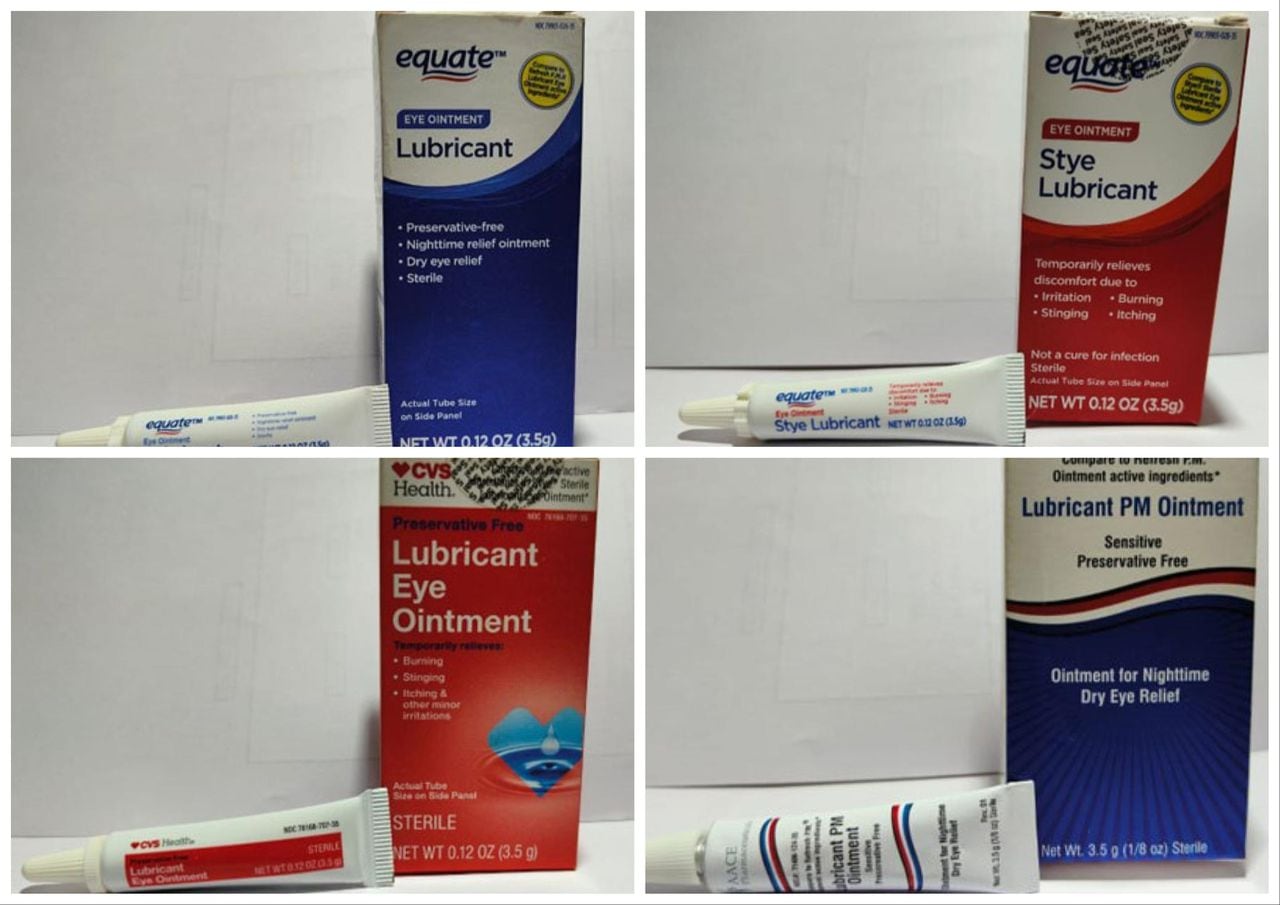
The FDA has issued a recall for four types of eyedrops sold at Walmart and CVS due to a lack of sterility assurance at the facility where they are made. The eyedrops, produced by Brassica Pharma Pvt. Ltd., are at risk of potential eye infections or related harm and should not be used. The recalled products should be returned to the retailer where they were purchased, and individuals can visit the FDA website for more information on the recall.
- FDA says you should stop using these eyedrops sold at Walmart and CVS MLive.com
- Popular eye ointments may not be sterile, recall warns CNN
- CVS, Walmart Eye Ointments Recalled For Potential Eye Infection Risk: What To Know Forbes
- Recall Alert: Eye Ointments Sold at Walmart, CVS May Pose Infection Risk Health.com
- Eye Ointments at CVS and Walmart Pulled Because of Infection Risk The New York Times
Reading Insights
Total Reads
0
Unique Readers
2
Time Saved
1 min
vs 2 min read
Condensed
74%
304 → 79 words
Want the full story? Read the original article
Read on MLive.com