FDA finds sterilization issues at maker of eye drops linked to deadly outbreak.
TL;DR Summary
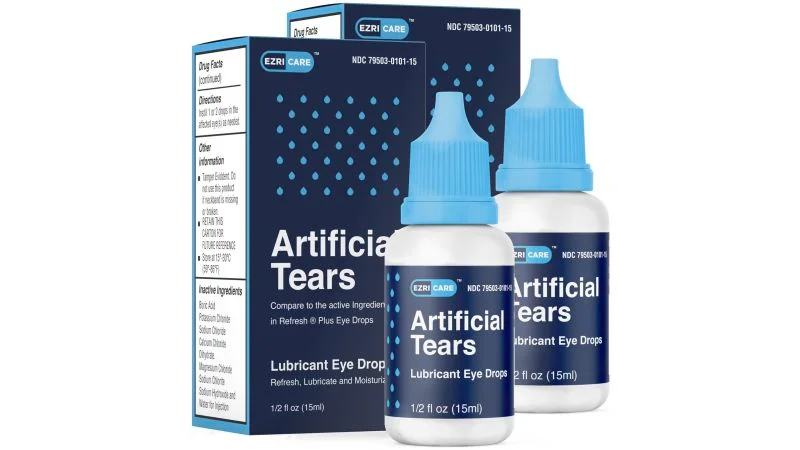
An FDA inspection found that Global Pharma Healthcare, the manufacturer of EzriCare Artificial Tears eye drops linked to an outbreak of serious bacterial infections in the US, did not follow proper protocol to prevent contamination of its products. The inspection resulted in 11 observations, including a "manufacturing process that lacked assurance of product sterility." The FDA continues to monitor the issue and is working with the CDC and the companies recalling affected products. Consumers are urged to stop using these products which may be harmful to their health.
- FDA inspection finds sterilization issues at recalled eye drop manufacturer's facility CNN
- Drug-Resistant Bacteria Tied to Eyedrops Can Spread Person to Person The New York Times
- Maker of eye drops linked to deadly outbreak flunks FDA inspection Ars Technica
- Chennai company recalls 50K tubes of eye drug in US The Tribune India
- What to Know About Bacteria and Eye Drops The New York Times
Reading Insights
Total Reads
0
Unique Readers
1
Time Saved
2 min
vs 3 min read
Condensed
82%
488 → 88 words
Want the full story? Read the original article
Read on CNN