FDA Denies Approval for Needle-Free EpiPen Alternative, Demands Further Study
TL;DR Summary
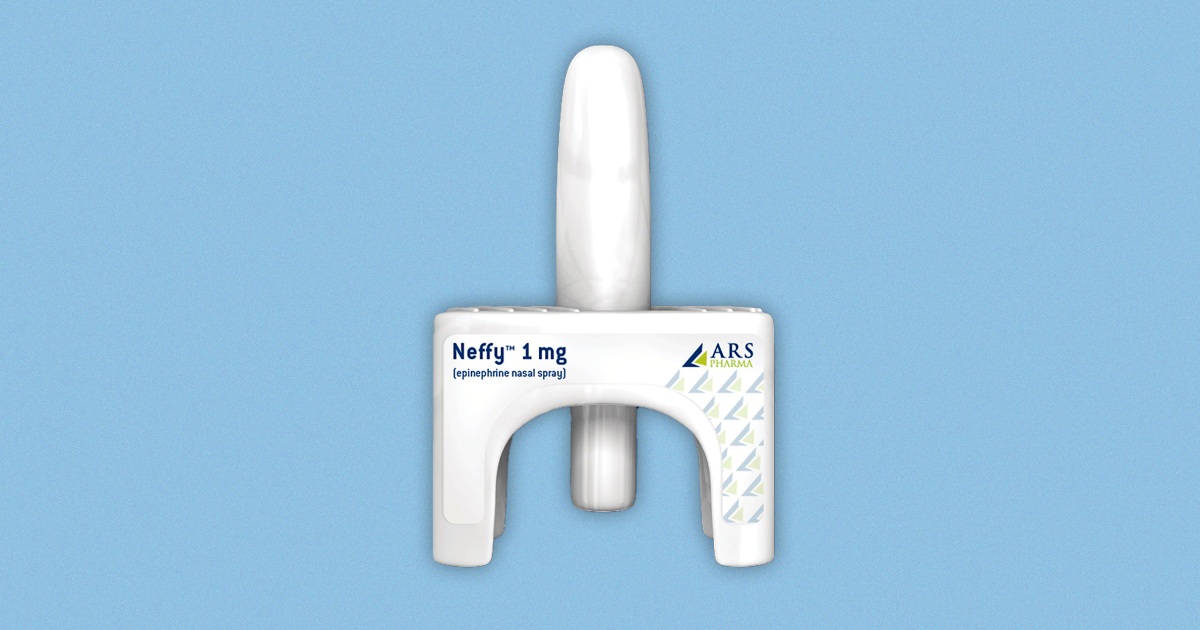
The FDA has rejected the approval of Neffy, an epinephrine nasal spray that would have been the first needle-free alternative to EpiPens. The agency has requested additional research to support approval, despite its advisory committee recommending the drug in May. Currently, all available epinephrine treatments require injection, posing a challenge for those afraid of needles. Concerns were raised about the lack of clinical data, particularly in patients experiencing anaphylaxis. The drugmaker plans to appeal the FDA's request and resubmit its application in 2024.
Reading Insights
Total Reads
0
Unique Readers
1
Time Saved
3 min
vs 4 min read
Condensed
87%
646 → 83 words
Want the full story? Read the original article
Read on NBC News