FDA Approves Groundbreaking Cell Therapy for Type 1 Diabetes
TL;DR Summary
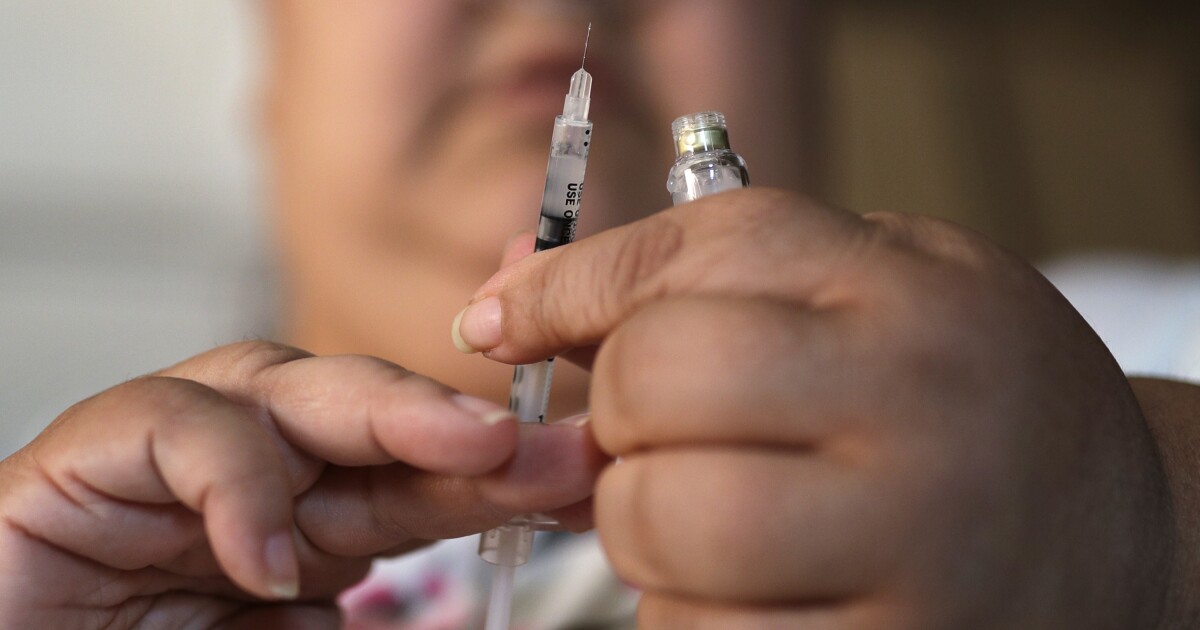
The FDA has approved Lantidra, a cellular therapy made from deceased donor pancreatic cells, to help adults with Type 1 diabetes maintain their target blood glucose levels. Lantidra aims to replace the insulin-producing beta cells in the pancreas, potentially eliminating the need for daily injections or infusions of insulin. Clinical studies have shown that a significant number of participants did not need insulin for one to five years or more after receiving Lantidra infusions. However, there were some side effects and serious adverse reactions reported, so patients are advised to discuss the benefits and risks with their doctors.
- FDA approves new cell therapy for type 1 diabetes WXYZ 7 Action News Detroit
- FDA blesses CellTrans' Lantidra, the first cell therapy for Type 1 diabetes FiercePharma
- FDA OKs Pancreatic Islet Cell Therapy for Type 1 Diabetes Medscape
- FDA Approves First Cell Therapy for Type 1 Diabetes BioSpace
- First cell therapy for type 1 diabetes approved for use in the US New Scientist
Reading Insights
Total Reads
0
Unique Readers
0
Time Saved
2 min
vs 3 min read
Condensed
83%
591 → 98 words
Want the full story? Read the original article
Read on WXYZ 7 Action News Detroit