FDA Approves Biannual HIV Prevention Shot for Enhanced Protection
TL;DR Summary
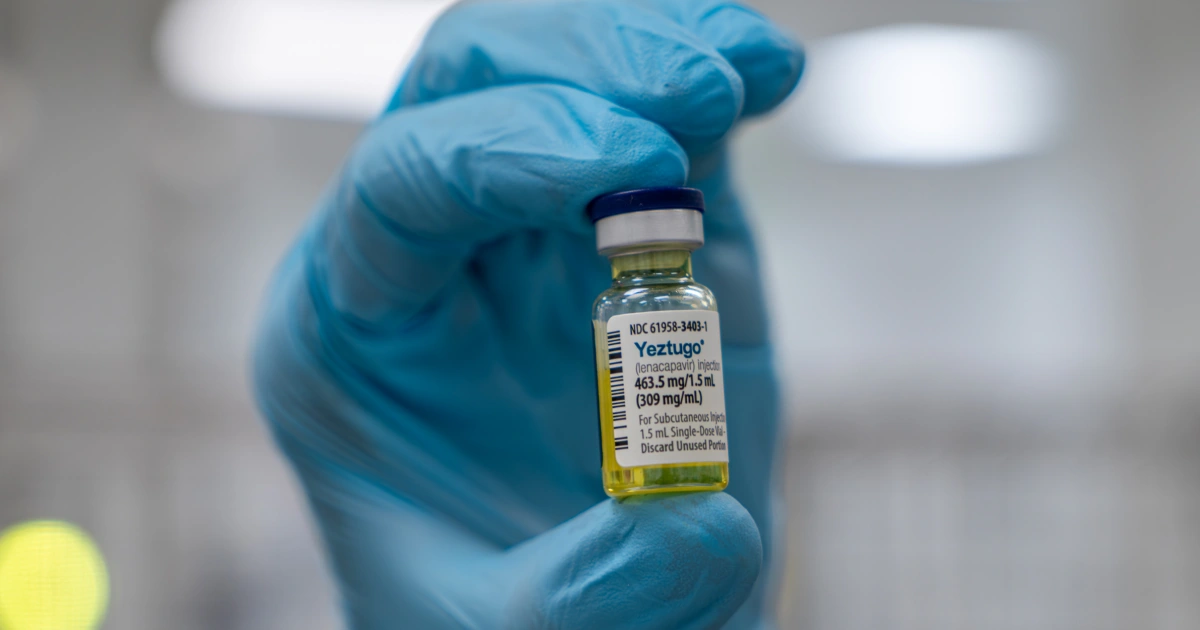
The FDA has approved Yeztugo, a long-acting HIV prevention drug by Gilead Sciences, which is injected twice a year and shows high efficacy in preventing HIV transmission, especially among high-risk populations. Despite its potential, challenges such as high cost, insurance coverage issues, and political funding cuts could hinder its widespread use and impact on reducing HIV rates in the US.
- FDA approves powerful HIV prevention drug: What to know about lenacapavir NBC News
- FDA approves Gilead's twice-yearly HIV prevention injection, offering a powerful and convenient new option CNBC
- HIV protection with just two shots a year: FDA approves Gilead drug statnews.com
- FDA approves new twice-yearly HIV shot. What to know USA Today
- Regulators Approve a Twice-Yearly Shot to Prevent H.I.V. Infection The New York Times
Reading Insights
Total Reads
0
Unique Readers
1
Time Saved
8 min
vs 8 min read
Condensed
96%
1,578 → 60 words
Want the full story? Read the original article
Read on NBC News