CDC and FDA Warn Against Using Certain Eye Products Due to Safety Concerns
TL;DR Summary
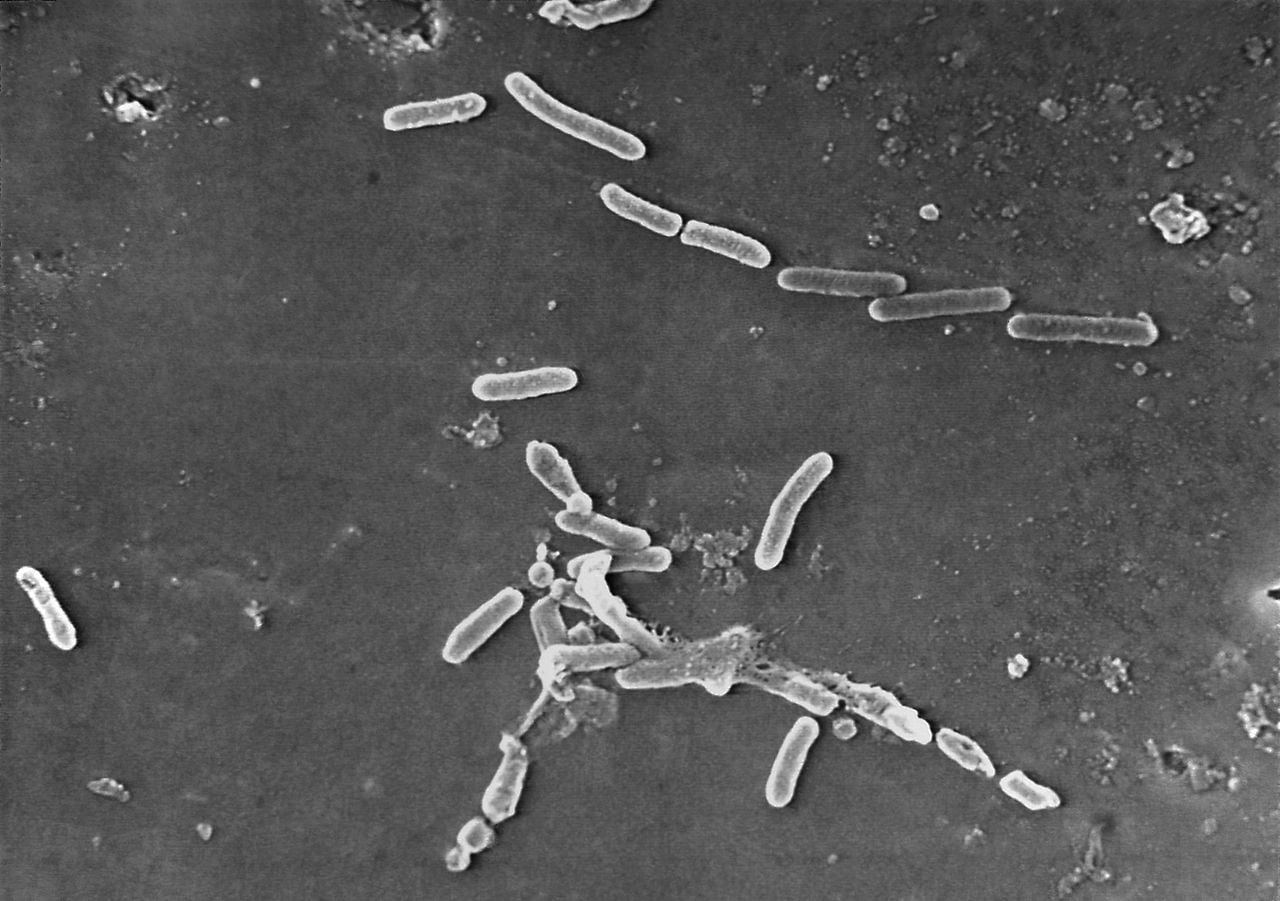
The Centers for Disease Control and Prevention (CDC) has warned people to stop using recalled eye products linked to an outbreak of Pseudomonas aeruginosa, a rare bacteria that has infected 68 patients in 16 states, resulting in three deaths, eight cases of vision loss, and four patients having their eyeballs removed. The recalled products are EzriCare Artificial Tears, Delsam Pharma Artificial Tears, and Delsam Pharma Artificial Eye Ointment, all of which are imported from India-based Global Pharma Healthcare Private Ltd. People are advised to seek immediate medical attention if they experience symptoms of an eye infection.
- Stop using these eye drops immediately, CDC warns AL.com
- Stop using these eye products linked to 3 deaths, removal of 4 eyeballs, CDC warns OregonLive
- FDA: Sterilization issues at company linked to eye infections | FOX 13 Seattle FOX 13 Seattle
- Eyedrops maker couldn't ensure factory was sterile, FDA says AOL
- An Eye Drop May Have Introduced Dangerous Bacteria to US, Expert Advises How to Prevent The Epoch Times
Reading Insights
Total Reads
0
Unique Readers
3
Time Saved
1 min
vs 2 min read
Condensed
69%
310 → 96 words
Want the full story? Read the original article
Read on AL.com