"Breakthrough Liver Disease Drug Marks New Era in Biopharma Innovation"
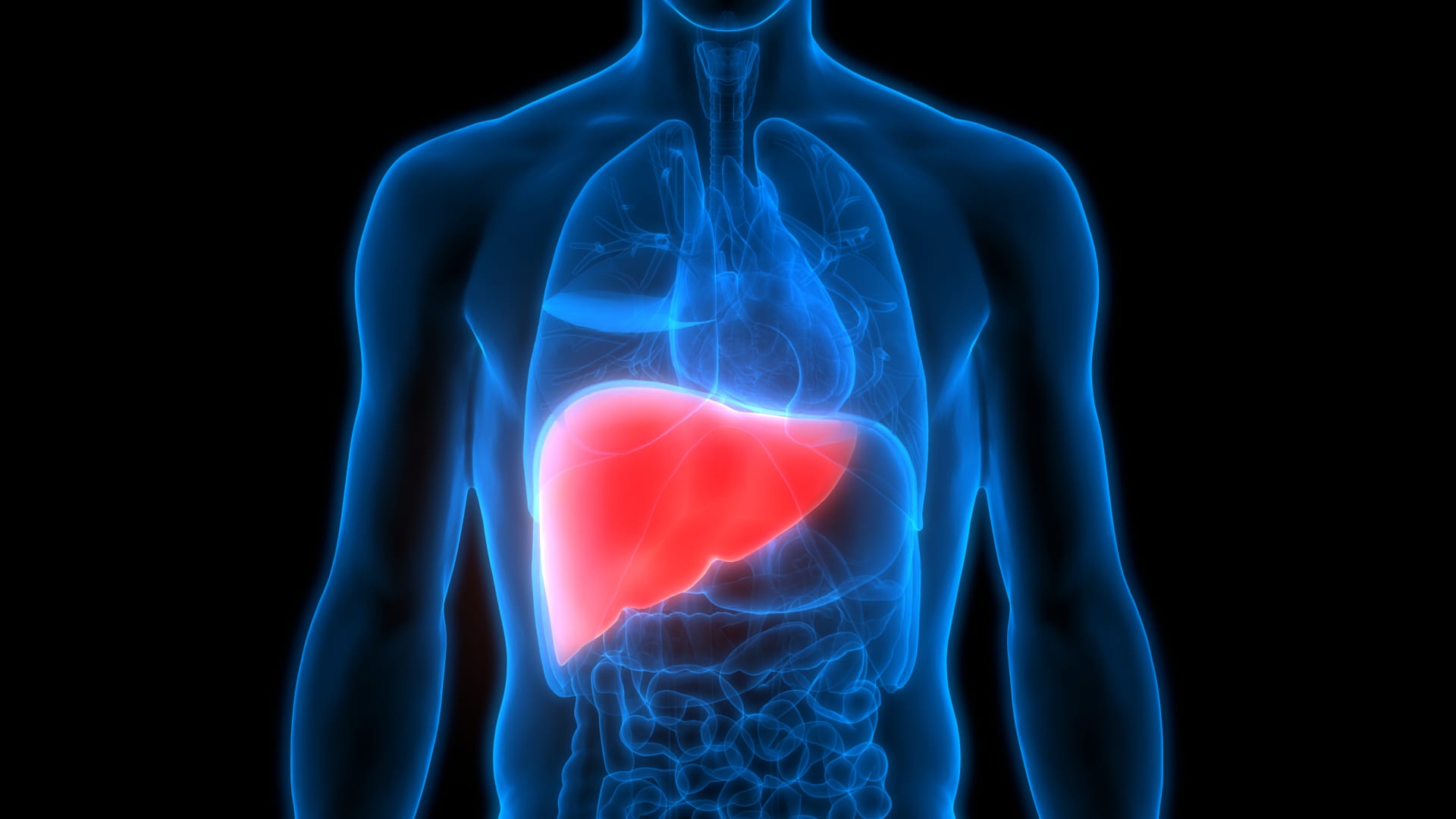
The FDA has approved Madrigal Pharmaceuticals' drug "Rezdiffra" for the treatment of nonalcoholic steatohepatitis (NASH), a common and serious liver disease affecting millions of Americans. The approval marks a significant milestone as it is the first-ever treatment for NASH, with a price tag of $47,400 per year. This decision opens the door for other companies developing NASH treatments, and several biotech firms are currently studying experimental drugs for the condition. Additionally, the use of GLP-1s, including Novo Nordisk's Wegovy and Eli Lilly's Zepbound, in treating NASH is being closely watched, as they could potentially dominate the market. The approval of Rezdiffra without the need for a liver biopsy could accelerate and broaden patient access to NASH treatments.
- Healthy Returns: The first drug for a common, deadly liver disease is here – and more are coming CNBC
- First US drug approved for a liver disease surging around the world Nature.com
- Ozempic Can't Do It All—A Potential New Blockbuster Is a Reminder of That The Wall Street Journal
- FDA approves first MASH drug: Madrigal's Rezdiffra breaks ground in notorious biopharma graveyard FiercePharma
- Madrigal lands MASH nod. What's next for biotechs in pursuit? Fierce Biotech
Reading Insights
0
5
6 min
vs 7 min read
90%
1,231 → 117 words
Want the full story? Read the original article
Read on CNBC