Redefining the Role of Metal Cocatalysts in Photocatalysis
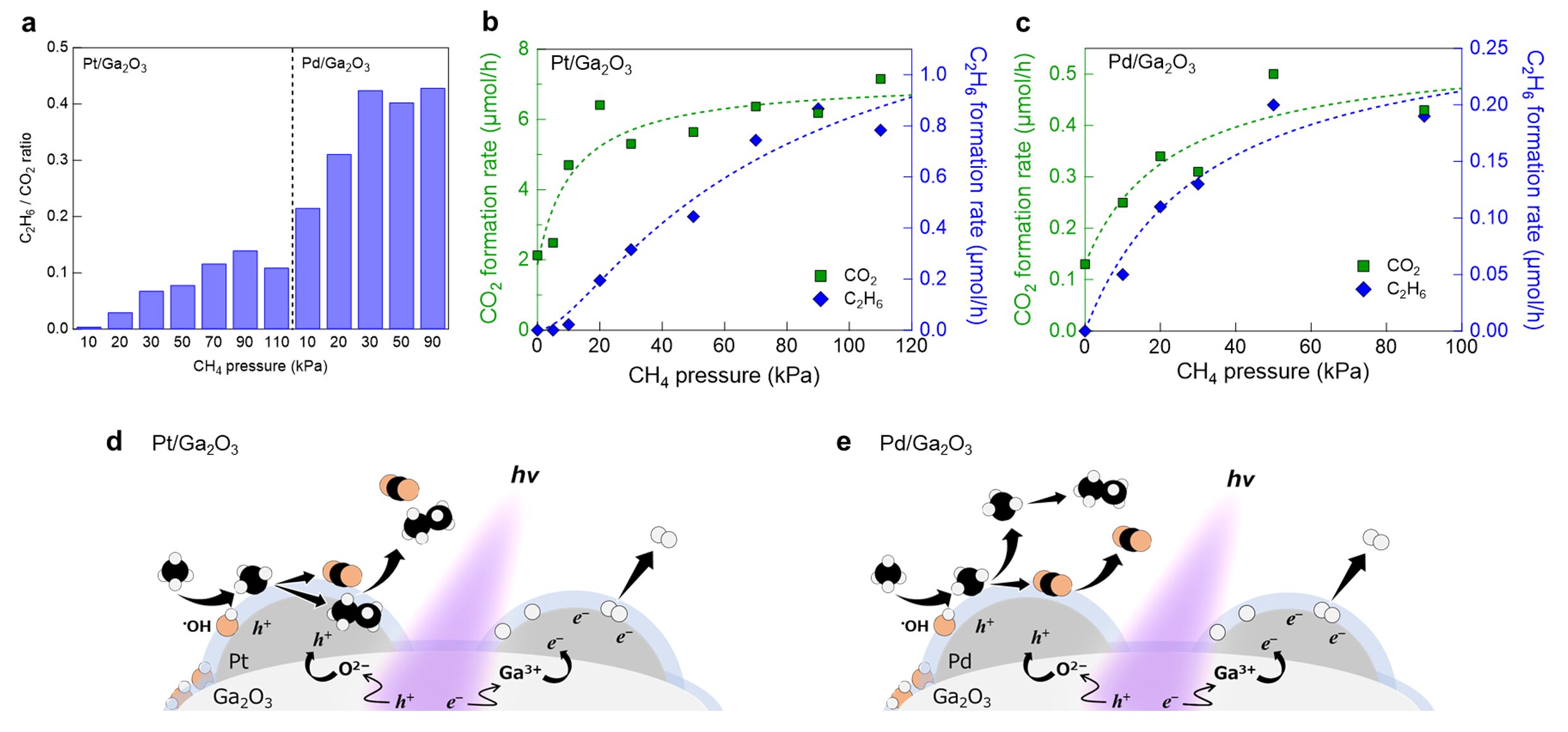
Researchers have discovered that metal cocatalysts loaded on a semiconductor photocatalyst play a crucial role in modulating surface oxidation kinetics and selectivity in the photocatalytic conversion of methane with water. The study found that platinum (Pt) cocatalysts predominantly promote the total oxidation of methane to carbon dioxide (CO2), while palladium (Pd) cocatalysts exhibit a higher selectivity for ethane (C2H6) formation. The research also revealed that metal cocatalysts act as reservoirs of photogenerated holes and effective reaction sites for methane oxidation. This new understanding challenges the conventional assumption that metal cocatalysts only accumulate photogenerated electrons and promote reduction reactions, highlighting their potential for controlling non-thermal oxidation reactions and advancing green technology.
