MDMA Therapy for PTSD Gains Momentum Towards FDA Approval
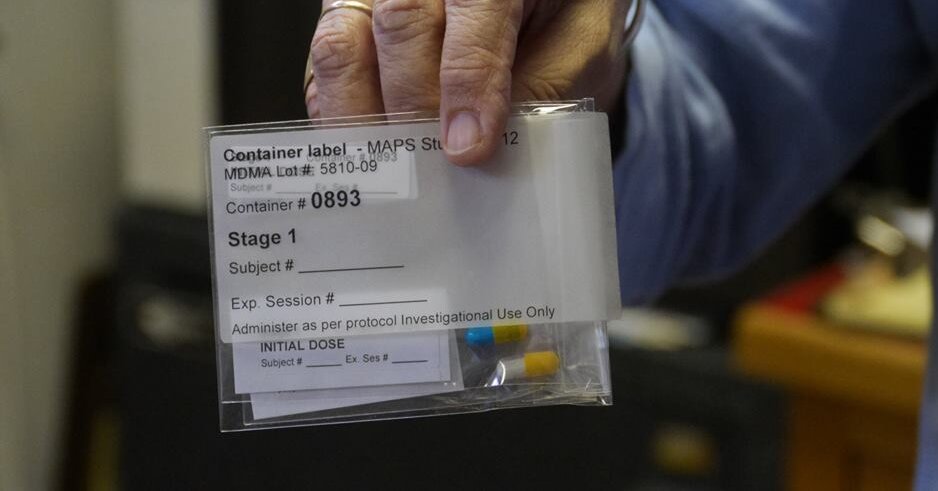
A Phase 3 clinical trial sponsored by the Multidisciplinary Association for Psychedelic Studies (MAPS) has shown that treatment with MDMA, also known as ecstasy, significantly reduced symptoms in patients with moderate to severe post-traumatic stress disorder (PTSD). The findings indicate that MDMA-assisted therapy could be considered for approval by the U.S. Food and Drug Administration (FDA) in 2024. The trial involved 104 participants who received either MDMA or a placebo, combined with talk therapy. MDMA was found to enhance therapy by reducing fear, threat, and negative emotions, allowing patients to process past trauma. The study also highlighted the safety and tolerability of MDMA treatment.