WHO and Gilead advance HIV prevention with new injectable options and global funding efforts
TL;DR Summary
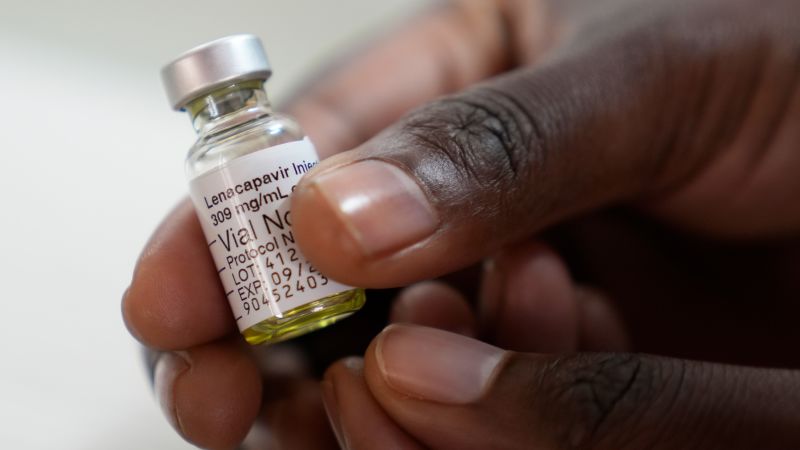
The WHO recommends the use of the long-acting HIV prevention injection lenacapavir, approved in the US for twice-yearly use, as part of global efforts to combat HIV, especially in high-risk groups. Despite its potential, funding cuts, particularly from the US, threaten to undermine HIV prevention programs worldwide, risking increased infections and deaths. Gilead Sciences has committed to supplying lenacapavir at no profit to improve access in low-income countries, but global resource gaps remain a major challenge in ending the HIV epidemic.
- WHO recommends twice-a-year HIV prevention shot as concern looms over funding for global HIV fight CNN
- WHO recommends injectable lenacapavir for HIV prevention World Health Organization (WHO)
- Gilead, Global Fund finalize plan to supply HIV prevention drug to poor countries Reuters
- Gilead partners with the Global Fund to support Yeztugo access strategy on heels of FDA nod Fierce Pharma
- HIV vaccine a closer reality with new HIV PrEP injection MSN
Reading Insights
Total Reads
0
Unique Readers
8
Time Saved
5 min
vs 6 min read
Condensed
93%
1,179 → 81 words
Want the full story? Read the original article
Read on CNN