Atorvastatin Recall Highlights FDA Inspection Challenges Abroad
TL;DR Summary
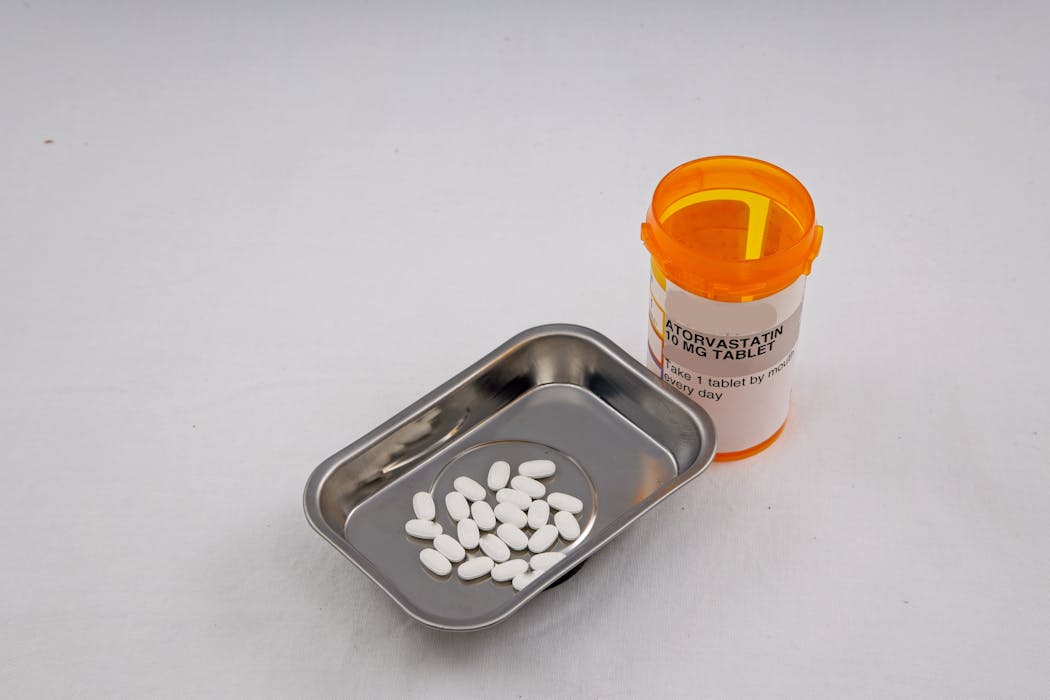
The recall of thousands of atorvastatin bottles due to manufacturing defects highlights ongoing issues with overseas drug production and FDA oversight, risking reduced drug efficacy and increased cardiovascular risks for millions of patients.
Reading Insights
Total Reads
0
Unique Readers
2
Time Saved
6 min
vs 7 min read
Condensed
97%
1,220 → 33 words
Want the full story? Read the original article
Read on Yahoo