"FDA Approves Dexcom's OTC iPhone-Enabled Glucose Monitor"
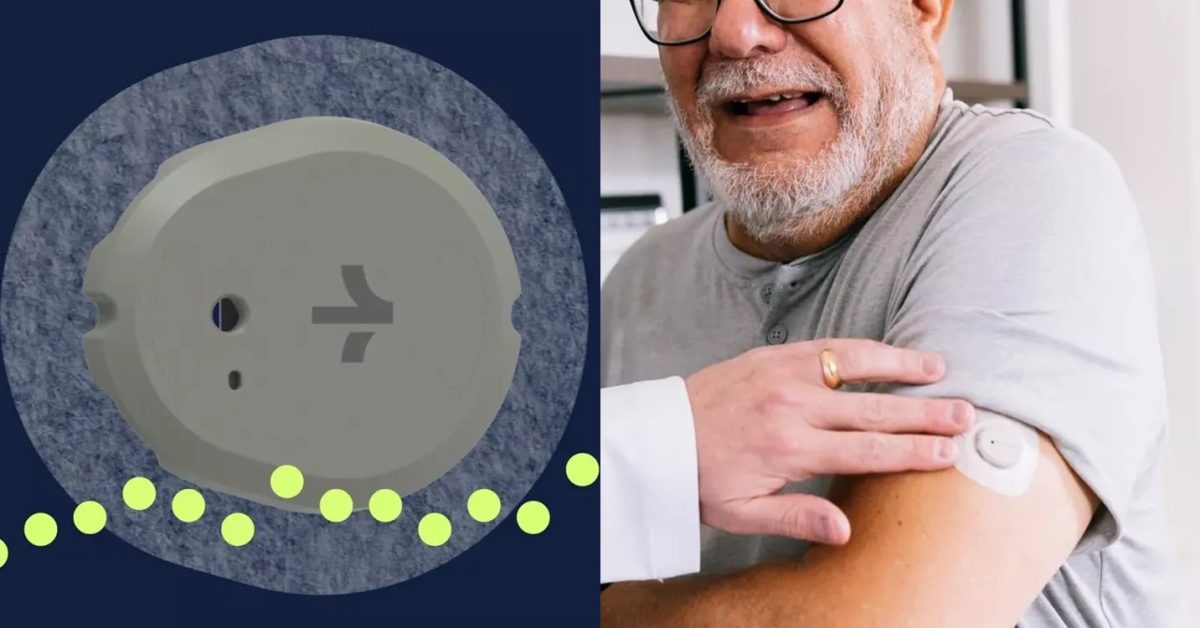
The FDA has approved Dexcom's Stelo Glucose Biosensor System, an iPhone-enabled blood glucose monitor that does not require a prescription, aimed at non-insulin dependent diabetics and those at risk of developing diabetes. The device uses a wearable sensor and smartphone app to continuously measure and display glucose values, with measurements taken automatically every 15 minutes. It is intended for individuals 18 years and older who do not use insulin, providing greater health equity by moving care and wellness into the home setting. The device is set to go on sale in the US this summer, offering a step towards the long-term goal of non-invasive blood sugar measurement.
- FDA approves first iPhone-enabled blood glucose monitor without a prescription 9to5Mac
- Dexcom's first-ever over-the-counter glucose monitor patch gets FDA clearance CNBC
- FDA Clears First OTC Continuous Glucose Monitoring, Dexcom Stelo Glucose Biosensor System Pharmacy Times
- FDA Clears Dexcom Stelo Sensor, First OTC Glucose Sensor in Agency History MD Magazine
- San Diego's Dexcom gets first FDA clearance for an over-the-counter wearable continuous glucose monitor The San Diego Union-Tribune
Reading Insights
1
2
1 min
vs 2 min read
72%
377 → 107 words
Want the full story? Read the original article
Read on 9to5Mac