Novo Nordisk Faces Legal and Investor Challenges Over Semaglutide Sales and Safety Concerns
TL;DR Summary
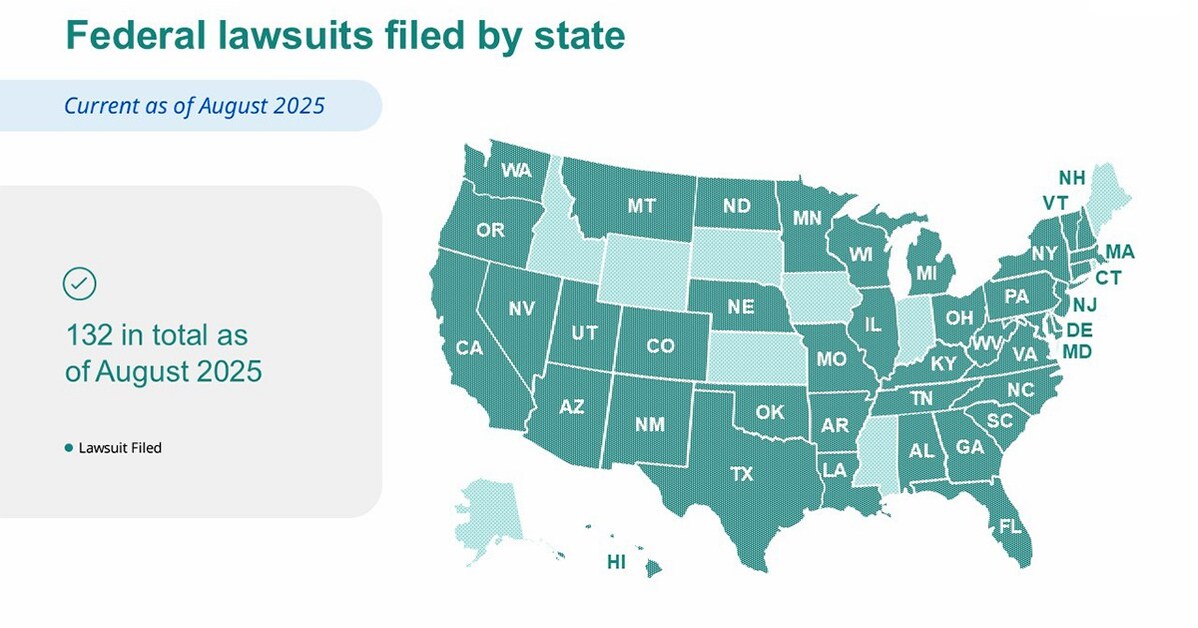
Novo Nordisk has filed over 130 lawsuits across 40 states to combat illegal marketing and sale of unsafe, non-FDA-approved compounded semaglutide products, which pose serious health risks due to illicit ingredients and misleading claims. The company is actively working to protect patients through legal action, education campaigns, and advocating for stricter enforcement against counterfeit and unapproved drugs.
- Novo Nordisk expands legal action to protect US patients from unsafe, non-FDA-approved compounded "semaglutide" PR Newswire
- Wegovy maker Novo hit with investor class action over revenue forecast cut Reuters
- Novo Nordisk faces investor lawsuit over 'false and misleading' semaglutide sales projections Fierce Pharma
- NOVO NORDISK A/S (NYSE: NVO) INVESTOR ALERT – Investors GlobeNewswire
- NVO Investors Have Opportunity to Lead Novo Nordisk A/S Securities Fraud Lawsuit with the Schall Law Firm Business Wire
Reading Insights
Total Reads
0
Unique Readers
1
Time Saved
5 min
vs 5 min read
Condensed
94%
982 → 57 words
Want the full story? Read the original article
Read on PR Newswire