FDA recalls 500,000 at-home COVID tests over bacterial contamination concerns.
TL;DR Summary
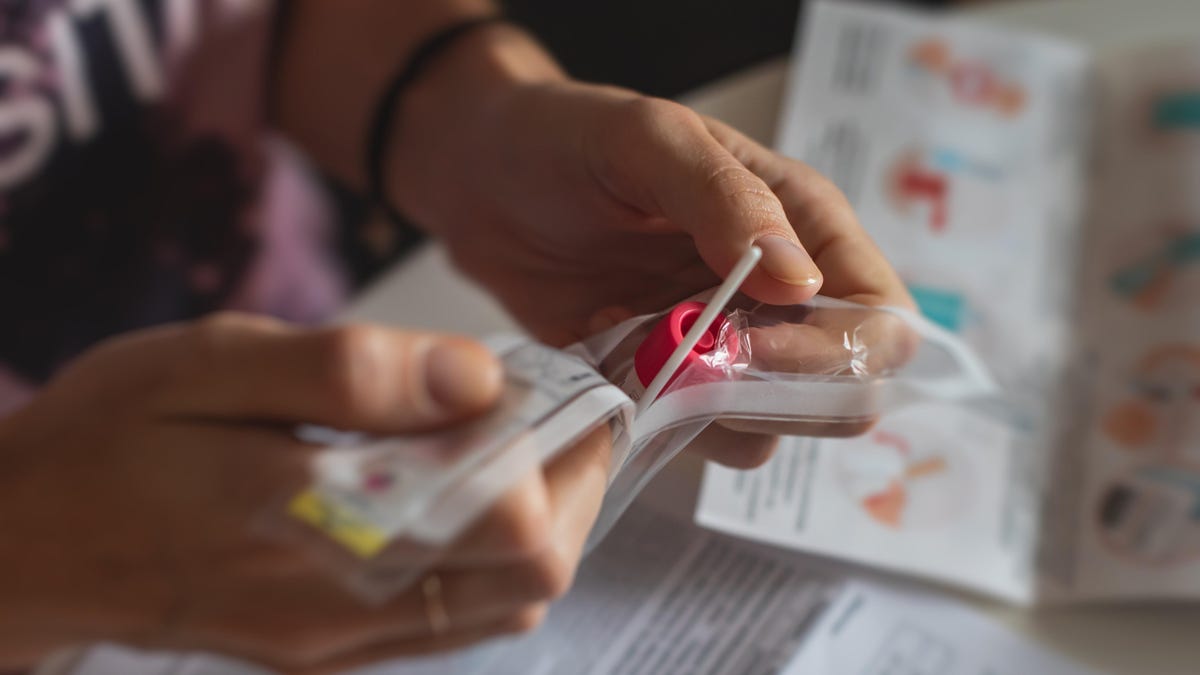
SD Biosensor is voluntarily recalling their at-home COVID-19 test kits in the U.S. due to potential bacterial contamination in the liquid inside the tube found in pouch number two. More than 500,000 of the kits were distributed to CVS drugstores and around 16,000 to Amazon. The recalled kits were distributed by Roche Diagnostics to distributors and retailers across the U.S. and were available over the counter. Consumers are urged not to use the recalled kits and to dispose of them properly. No official reports of illness have been reported.
- These Recalled COVID Tests May Be Contaminated With Bacteria Lifehacker
- FDA recalls brand of COVID-19 at-home test WFAA
- COVID test recall: FDA warns of bacterial contamination in some Pilot COVID-19 At-Home Tests from Roche and SD Biosensor CBS News
- FDA: Throw out Pilot COVID tests, potentially harmful bacteria CBS Chicago
- Roche, SD Biosensor recall 500000 at-home COVID tests due to bacteria risks FierceBiotech
Reading Insights
Total Reads
0
Unique Readers
0
Time Saved
2 min
vs 3 min read
Condensed
79%
428 → 89 words
Want the full story? Read the original article
Read on Lifehacker