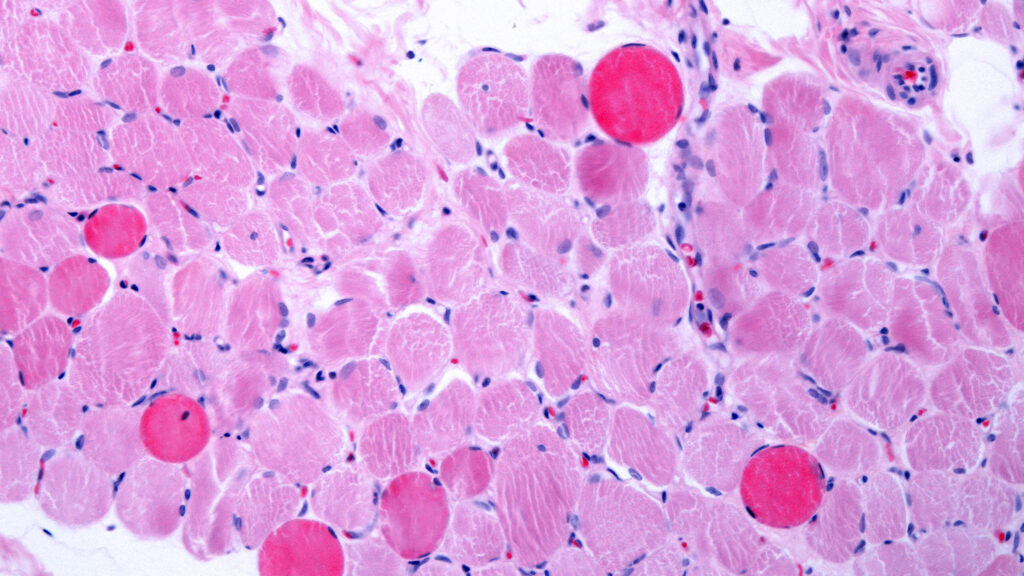
Second Death in Duchenne Gene Therapy Sparks Safety Concerns and Trial Halt
Following the death of a second patient treated with Elevidys for Duchenne muscular dystrophy, families and advocates are calling for greater transparency, improved safety data collection, and stronger regulatory oversight to ensure patient safety and informed decision-making in the use of this high-cost gene therapy.
